Question: A solid sphere (of radius R and density p ) made of substance A (of molecular weight M ) is suspended in a liquid B.
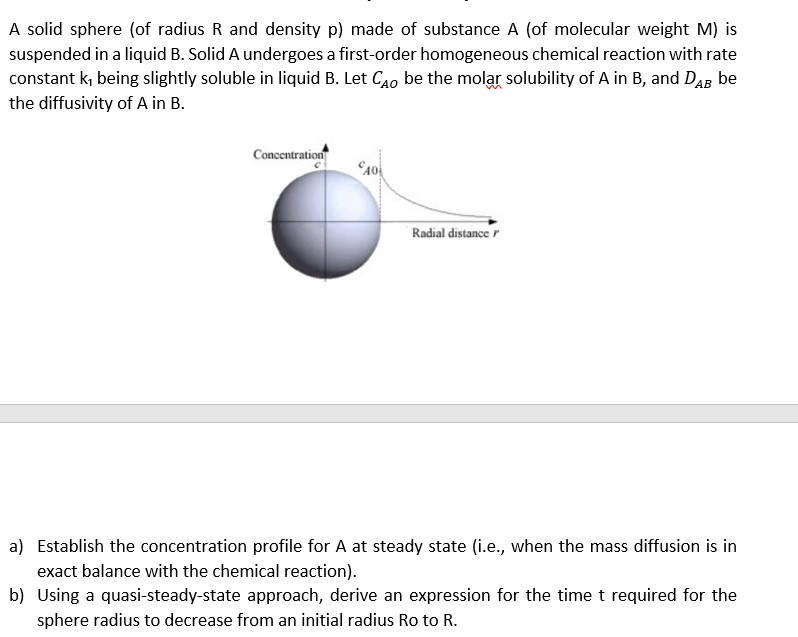
A solid sphere (of radius R and density p ) made of substance A (of molecular weight M ) is suspended in a liquid B. Solid A undergoes a first-order homogeneous chemical reaction with rate constant k1 being slightly soluble in liquid B. Let CAO be the molar solubility of A in B, and DAB be the diffusivity of A in B. a) Establish the concentration profile for A at steady state (i.e., when the mass diffusion is in exact balance with the chemical reaction). b) Using a quasi-steady-state approach, derive an expression for the time trequired for the sphere radius to decrease from an initial radius Ro to R
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


