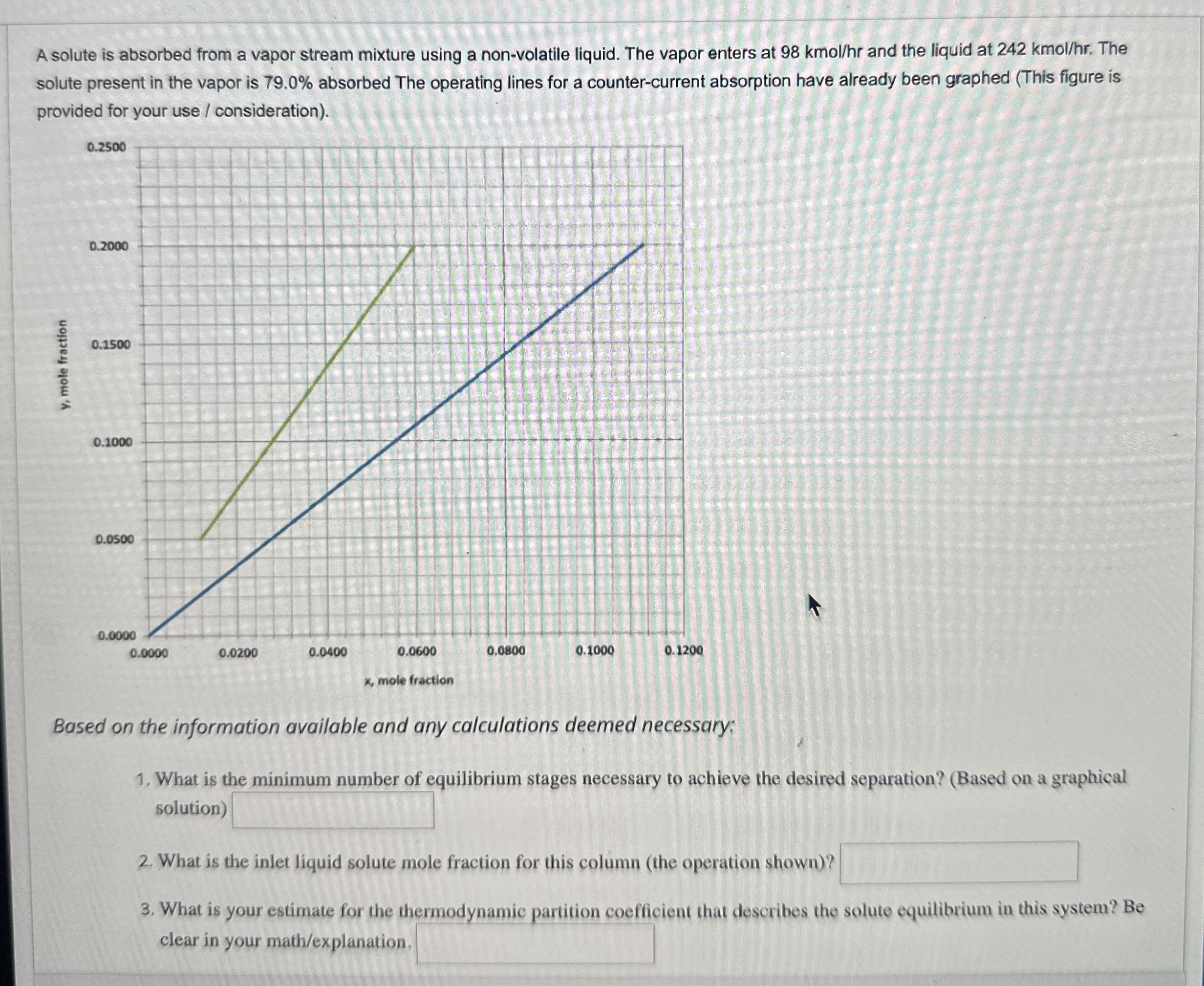
Question: A solute is absorbed from a vapor stream mixture using a non - volatile liquid. The vapor enters at 9 8 kmo l h r
A solute is absorbed from a vapor stream mixture using a nonvolatile liquid. The vapor enters at kmo and the liquid at kmo The solute present in the vapor is absorbed The operating lines for a countercurrent absorption have already been graphed This figure is provided for your use consideration
Based on the information available and any calculations deemed necessary:
What is the minimum number of equilibrium stages necessary to achieve the desired separation? Based on a graphical solution
What is the inlet liquid solute mole fraction for this column the operation shown
What is your estimate for the thermodynamic partition coefficient that describes the solute equilibrium in this system? Be clear in your mathexplanation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


