Question: A solution at pH 7 . 0 contains C u 2 + and N H 3 . a . What is the ratio C u
A solution at pH contains and
a What is the ratio
b What is the ratio
c Are there any hydroxo complexes of present at concentrations greater
than that of
d If which complex willumbe present at the great
est concentration?
e For a system with TOTCu and at what are and
when first becomes the dominant species?
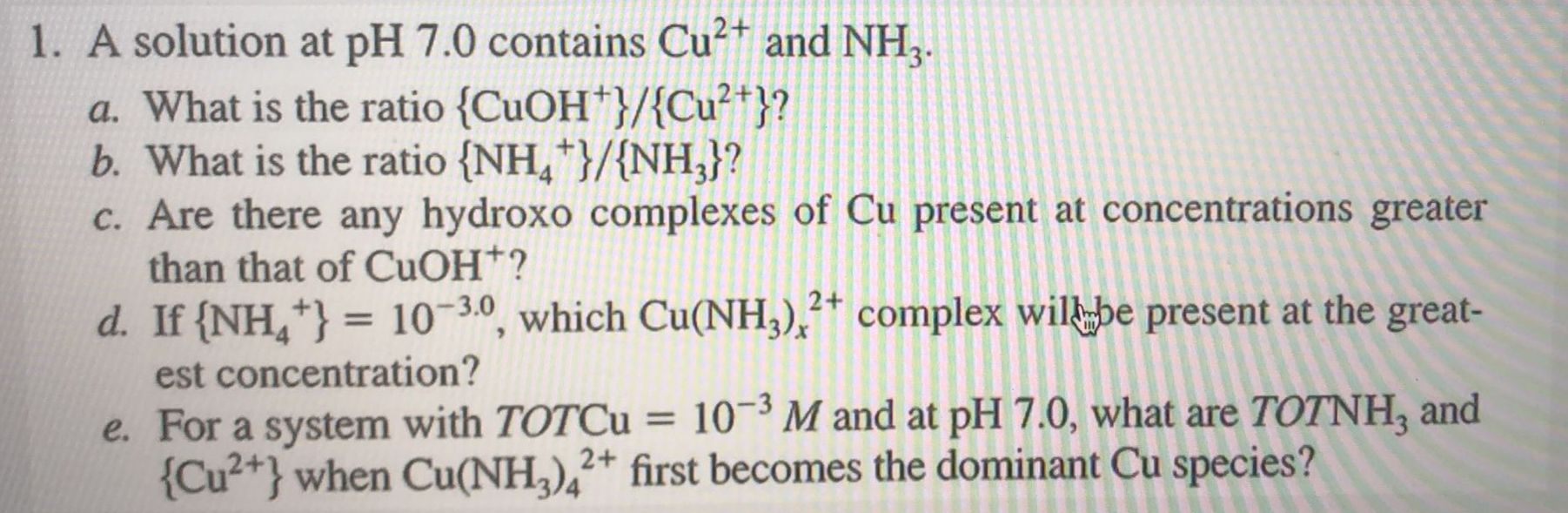
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


