Question: A solution at pH7 contains a weak acid, HA, which has a pKa of 6.5. Which of the following is closest to the molar ratio
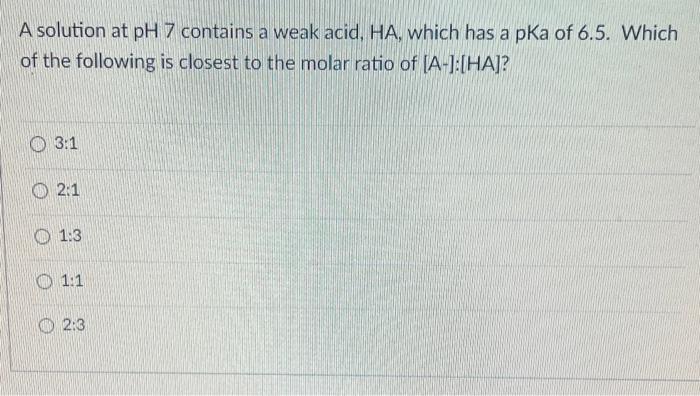
A solution at pH7 contains a weak acid, HA, which has a pKa of 6.5. Which of the following is closest to the molar ratio of [A-]:[HA]? 3:1 2:1 1:3 1:1 2:3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


