Question: Classification of Elements Properties Chen 340 Class 12023 Generally no luster No conduction Solids, liquids or gases. Classification Metals Metalloids Non-Metals Interpretation Close spacing. 30

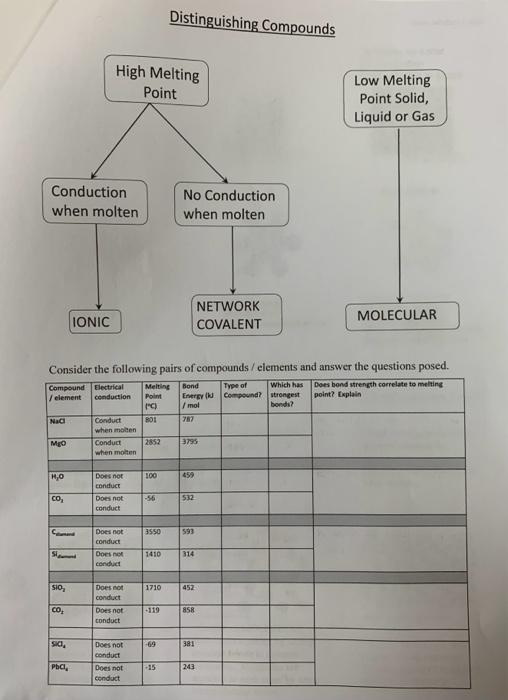
Classification of Elements Properties Chen 340 Class 12023 Generally no luster No conduction Solids, liquids or gases. Classification Metals Metalloids Non-Metals Interpretation Close spacing. 30 network of metallic bonds hold atoms together Widerspacing.3DnetworkofcovalentbondsholdatomstogetherGroupsofatomsheldtogetherbycovalentbondsinmolecules.Weakintermolecularforcesholdmoleculestogether Types of Compounds Classification Molecular Network Covalent Solids Properties - All high melting point - Low melting point solids, - High melting point solids SOLIDS at room temperature liquids or gases. at room temperature. - Conduct electricity - Do not conduct electricity when - Do nas conduct electricity when liquid (molten, fused). liquid. when molien. Interpretation - Consist of MOLECUL.FS - - Consist of IONS held atoms held together by strong - Consist of ATOMS held together by an extensive covalent bonds in discrete together by an extensive network of jonic molecules. Molecules are 3-D network of COVALENT interactions (SIRONG) attracted to each other by weak BONDS (STRONG) intermolecular forces. Consider the following pairs of compounds / elements and answer the questions posed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


