Question: A solution is made by adding 23.1 mL of concentrated nitric acid (70.4 wt%, density 1.42 g/mL) to some water in a volumetric flask, and
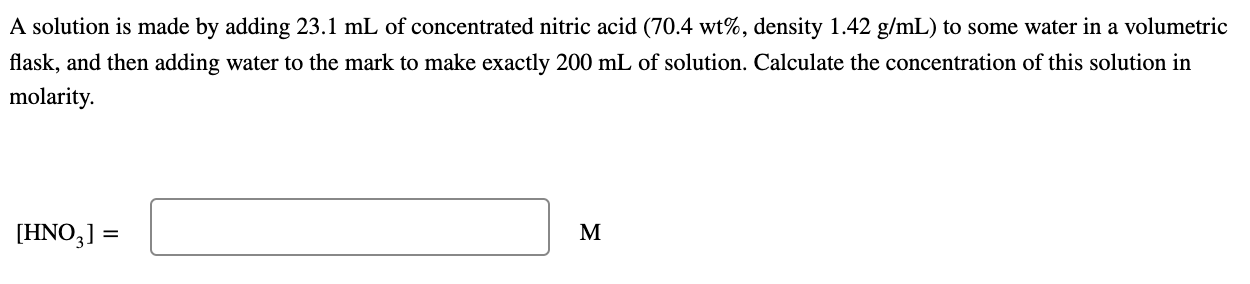
A solution is made by adding 23.1 mL of concentrated nitric acid (70.4 wt%, density 1.42 g/mL) to some water in a volumetric flask, and then adding water to the mark to make exactly 200 mL of solution. Calculate the concentration of this solution in molarity. [HNO3) = M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


