Question: A solution is made by dissolving 0.606mol of nonelectrolyte solute in 795g of benzene. Calculate the freezing point, Tf, and poiling point, Tb, of the

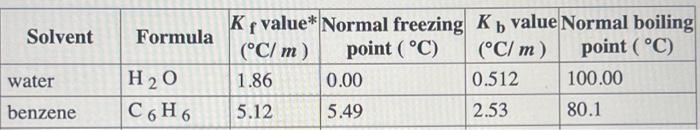
A solution is made by dissolving 0.606mol of nonelectrolyte solute in 795g of benzene. Calculate the freezing point, Tf, and poiling point, Tb, of the solution. Constants can be found in the table of colligative constants. Tf= \begin{tabular}{|l|l|l|l|l|l|} \hline \multicolumn{1}{|c|}{ Solvent } & Formula & Kfvalue*(C/m) & Normalfreezingpoint(C) & Kbvalue(C/m) & Normalboilingpoint(C) \\ \hline water & H2O & 1.86 & 0.00 & 0.512 & 100.00 \\ \hline benzene & C6H6 & 5.12 & 5.49 & 2.53 & 80.1 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


