Question: A solution is prepared by dissolving 3 x 10-3 mol PbC1, (s) in 1L of hot water. a) Write the correct balanced equilibrium reaction b)
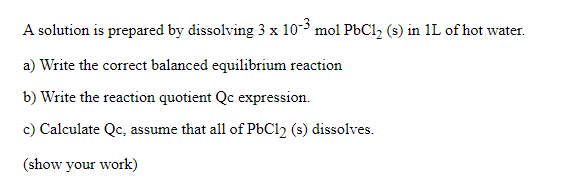
A solution is prepared by dissolving 3 x 10-3 mol PbC1, (s) in 1L of hot water. a) Write the correct balanced equilibrium reaction b) Write the reaction quotient Qc expression. c) Calculate Qc, assume that all of PbCl2 (5) dissolves. (show your work)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


