Question: A solution made by dissolving 150.0g of an unknown compound in 365.0mL of benzene to make a solution which has a freezing point 18.6C lower
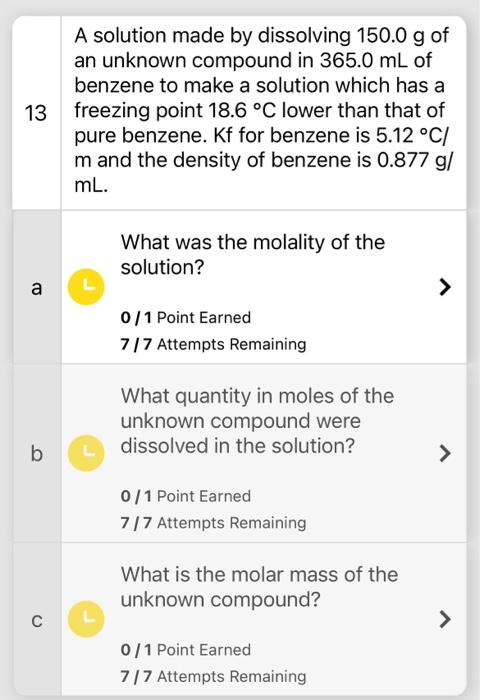
A solution made by dissolving 150.0g of an unknown compound in 365.0mL of benzene to make a solution which has a freezing point 18.6C lower than that of pure benzene. Kf for benzene is 5.12C/ m and the density of benzene is 0.877g/ mL. What was the molality of the solution? 0/1 Point Earned 7/7 Attempts Remaining What quantity in moles of the unknown compound were dissolved in the solution? 0/1 Point Earned 7/7 Attempts Remaining What is the molar mass of the unknown compound? 0/1 Point Earned 7/7 Attempts Remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


