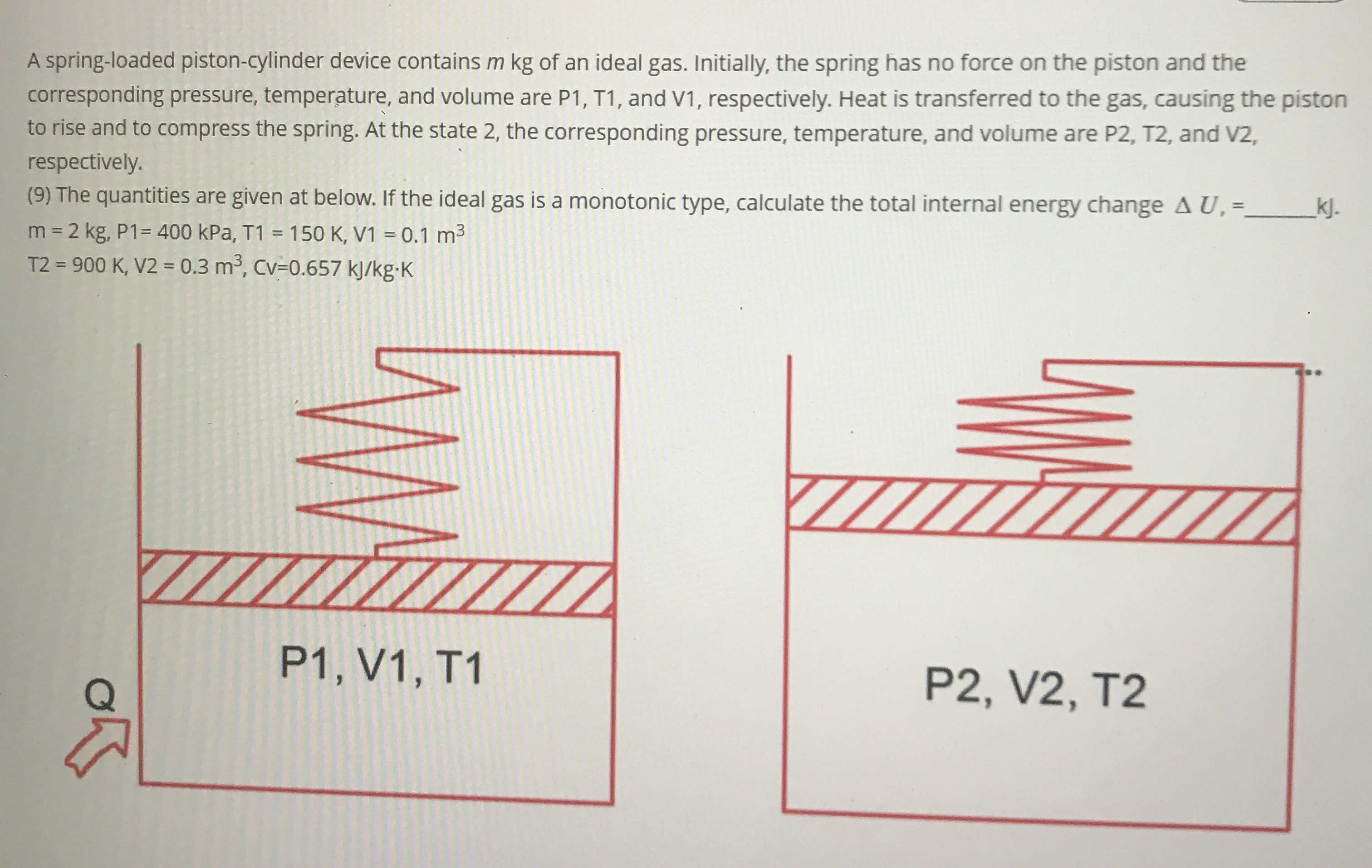
Question: A spring - loaded piston - cylinder device contains m k g of an ideal gas. Initially, the spring has no force on the piston
A springloaded pistoncylinder device contains of an ideal gas. Initially, the spring has no force on the piston and the corresponding pressure, temperature, and volume are P T and V respectively. Heat is transferred to the gas, causing the piston to rise and to compress the spring. At the state the corresponding pressure, temperature, and volume are and V respectively.
The quantities are given at below. If the ideal gas is a monotonic type, calculate the total internal energy change
kPa,
kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


