Question: QUESTION 2 ( PO 1 , CO 1 , C 2 ) 1 kg of ideal gas, initially at 1 0 0 kPa and 3
QUESTION
PO CO C
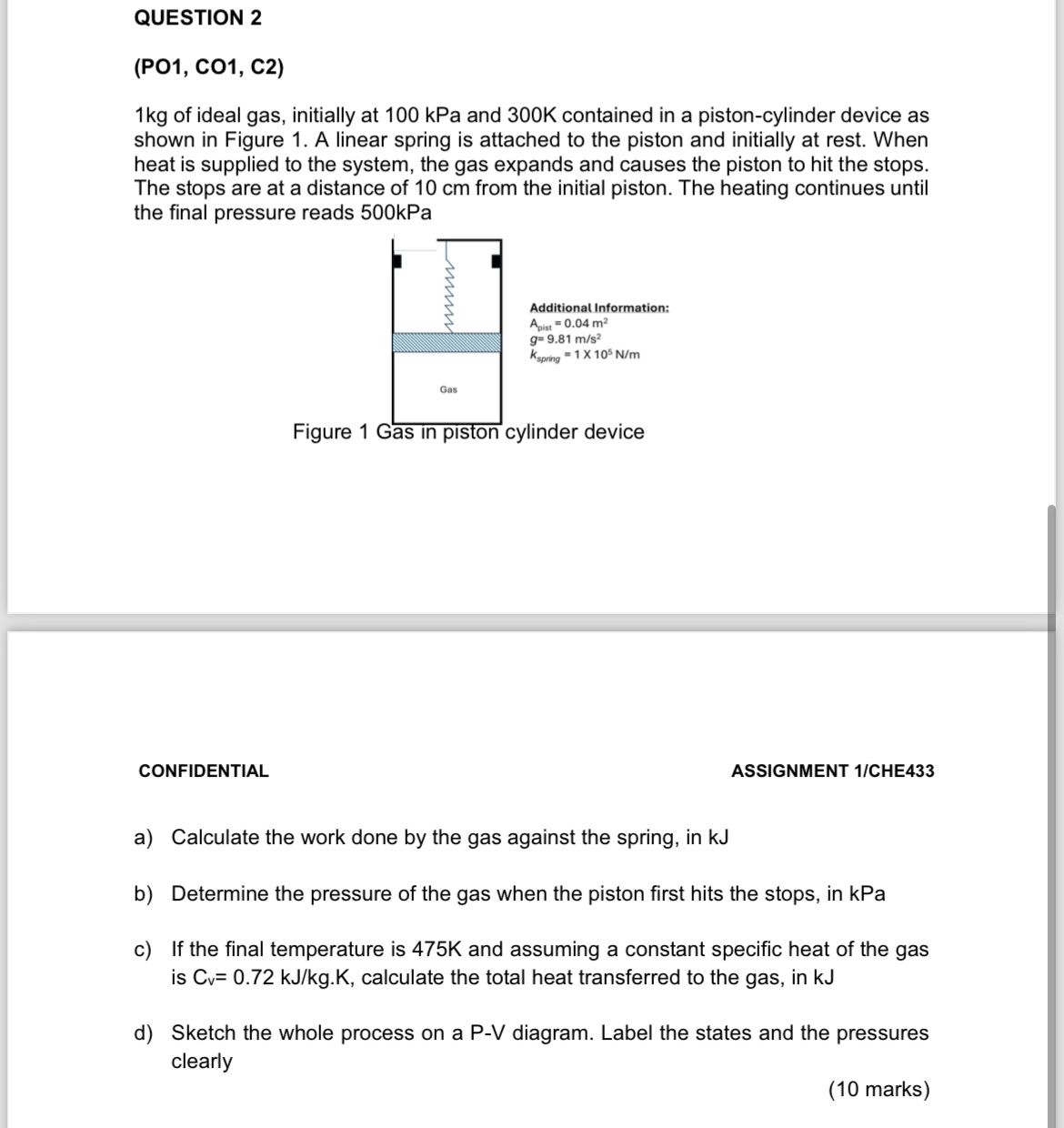
kg of ideal gas, initially at kPa and K contained in a pistoncylinder device as shown in Figure A linear spring is attached to the piston and initially at rest. When heat is supplied to the system, the gas expands and causes the piston to hit the stops. The stops are at a distance of cm from the initial piston. The heating continues until the final pressure reads kPa
Figure
CONFIDENTIAL
a Calculate the work done by the gas against the spring, in kJ
b Determine the pressure of the gas when the piston first hits the stops, in kPa
c If the final temperature is K and assuming a constant specific heat of the gas is mathrmCmathrmvmathrm~kJmathrmkgmathrmK calculate the total heat transferred to the gas, in kJ
d Sketch the whole process on a PV diagram. Label the states and the pressures clearly
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


