Question: A stock solution is made by dissolving 7 . 0 0 g of cobalt ( II ) nitrate hexahydrate into enough water to make 5
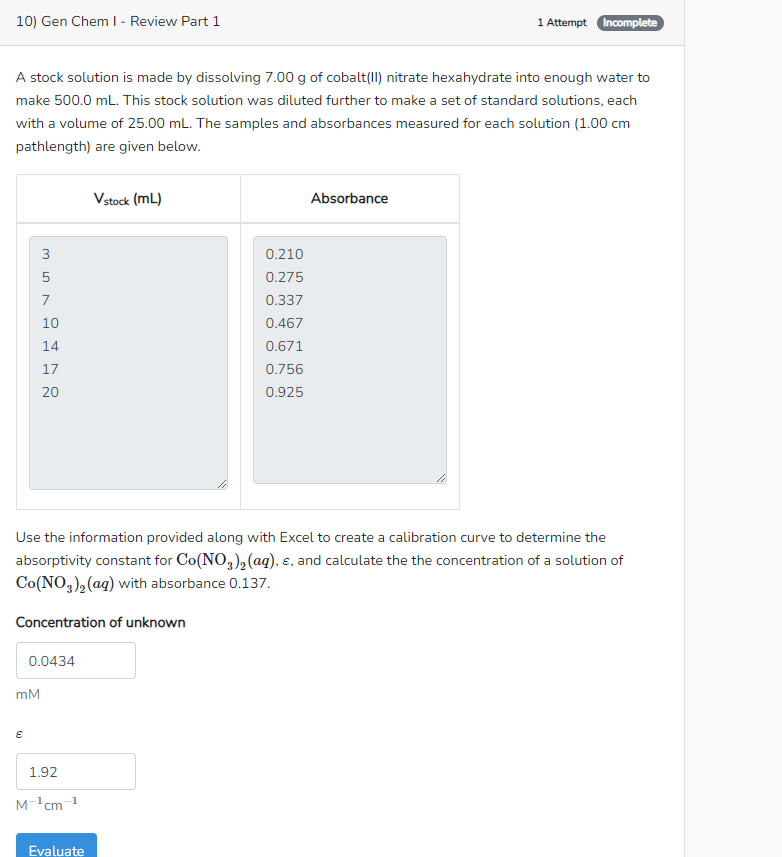
A stock solution is made by dissolving of cobaltII nitrate hexahydrate into enough water to
make This stock solution was diluted further to make a set of standard solutions, each
with a volume of The samples and absorbances measured for each solution
pathlength are given below.
Use the information provided along with Excel to create a calibration curve to determine the
absorptivity constant for and calculate the the concentration of a solution of
with absorbance Calculate the following
Concentration of unknown
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


