Question: A student has a sample containing 1.94 x 1026 copper (1) iodide molecules. How many grams of copper (I) iodide does the student have?
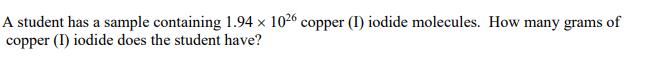
A student has a sample containing 1.94 x 1026 copper (1) iodide molecules. How many grams of copper (I) iodide does the student have?
Step by Step Solution
3.31 Rating (154 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


