Question: A student, Ken, is given a mixture containing two carbonate compounds. The mixture includes MgCO3 and (NH4)2CO3. The mixture is 65.95%CO3 is by mass. What
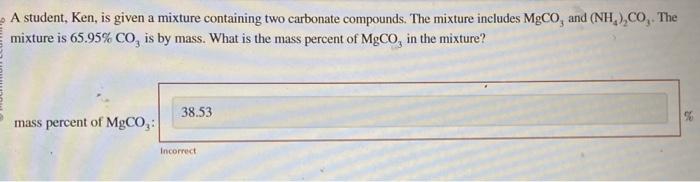

A student, Ken, is given a mixture containing two carbonate compounds. The mixture includes MgCO3 and (NH4)2CO3. The mixture is 65.95%CO3 is by mass. What is the mass percent of MgCO3 in the mixture? A mixture of 127.7g of Cl and 27.9g of P reacts completely to form PCl3 and PCl5. Find the mass of PCl5 produced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


