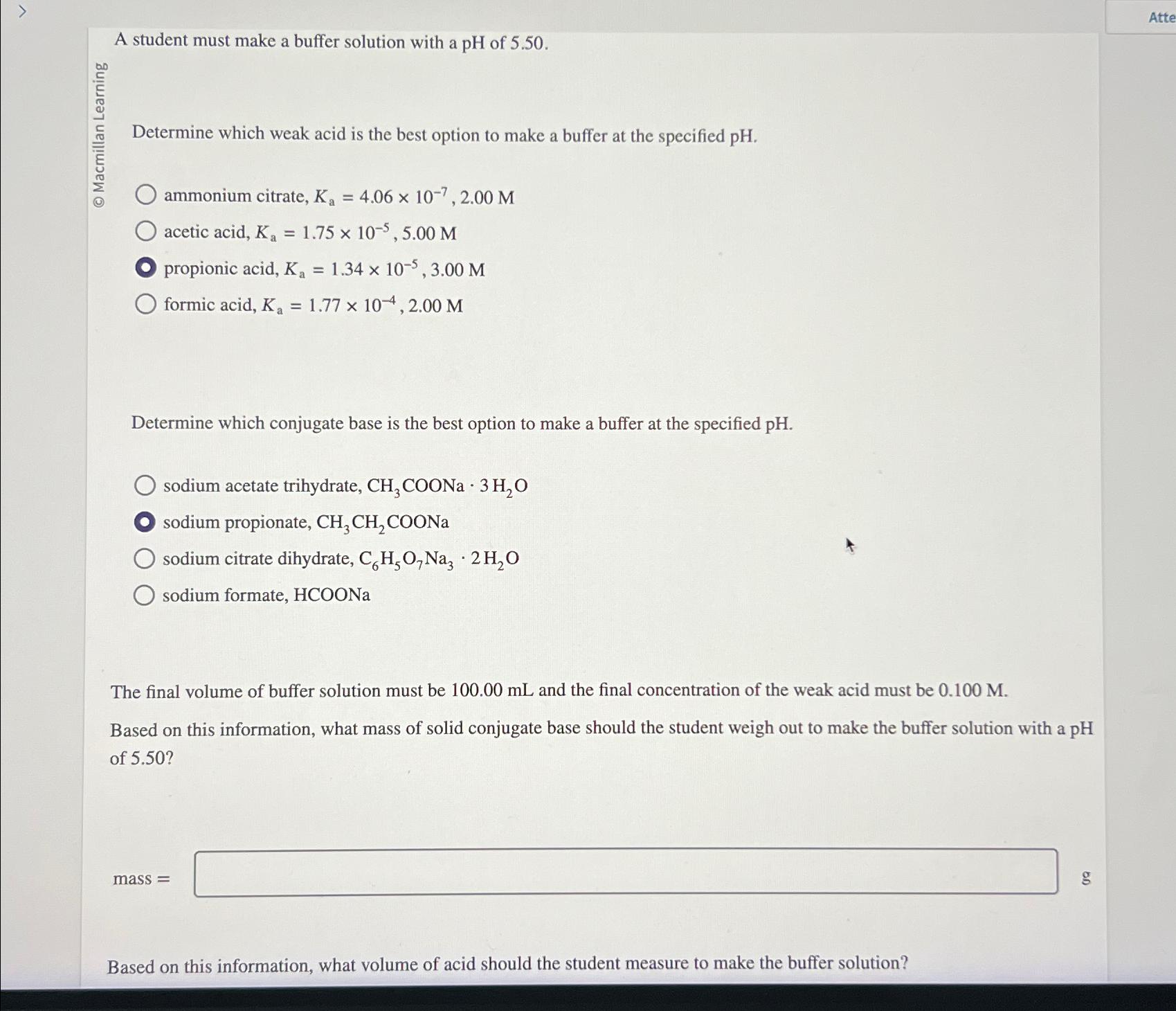
Question: A student must make a buffer solution with a p H of 5 . 5 0 . Determine which weak acid is the best option
A student must make a buffer solution with a of
Determine which weak acid is the best option to make a buffer at the specified
ammonium citrate,
acetic acid,
propionic acid,
formic acid,
Determine which conjugate base is the best option to make a buffer at the specified pH
sodium acetate trihydrate,
sodium propionate,
sodium citrate dihydrate,
sodium formate,
The final volume of buffer solution must be and the final concentration of the weak acid must be
Based on this information, what mass of solid conjugate base should the student weigh out to make the buffer solution with a pH of
mass
g
Based on this information, what volume of acid should the student measure to make the buffer solution?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


