Question: A student runs two experiments with a constant-volume bomb calorimeter containing 1400. g of water (see sketch at rj'ghr) . SU|-[-EI~|- thermometer First, a 5.000
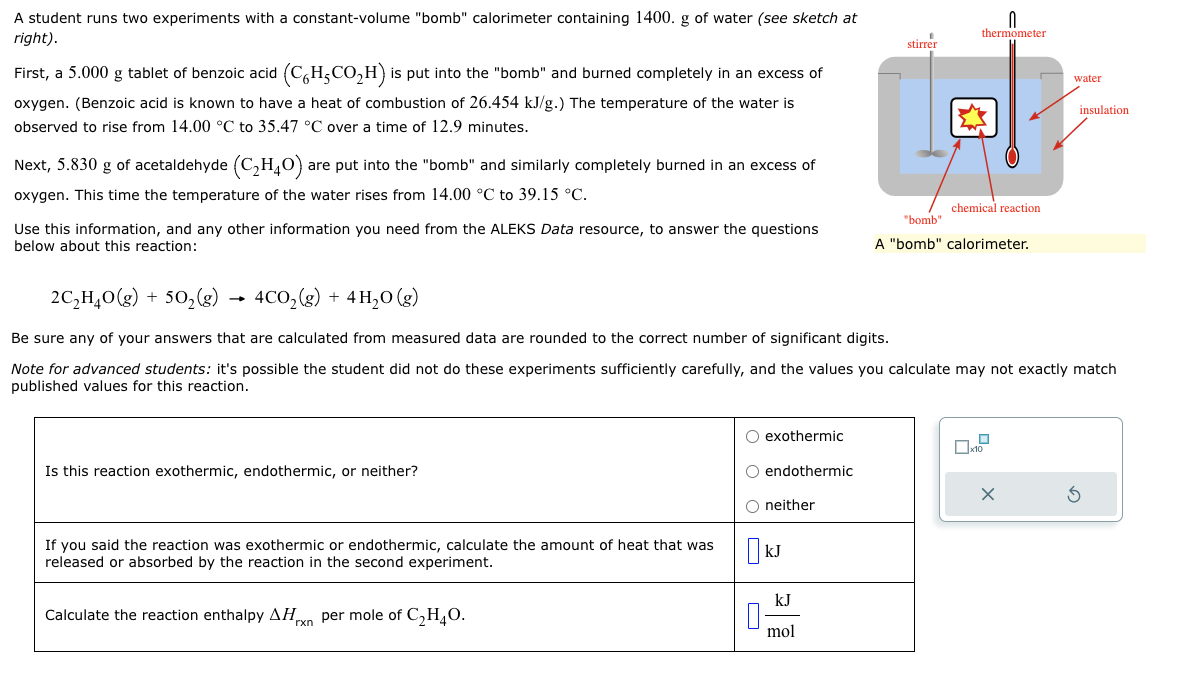

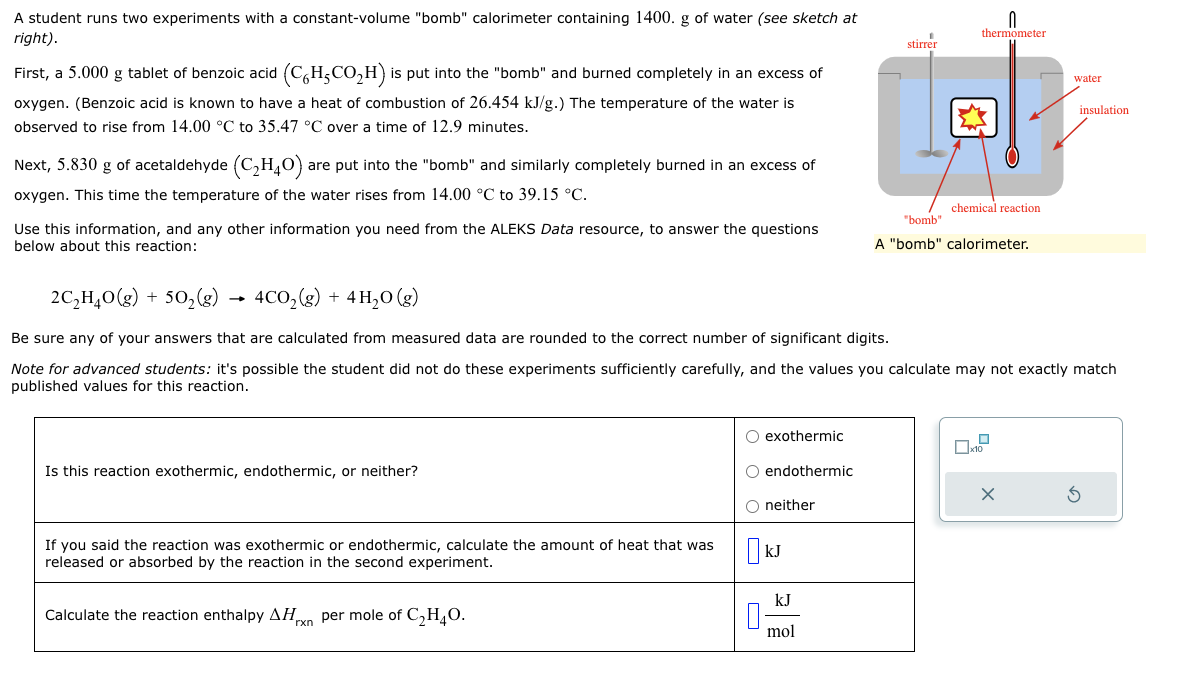

A student runs two experiments with a constant-volume "bomb" calorimeter containing 1400. g of water (see sketch at rj'ghr) . SU|-[-EI~|- thermometer First, a 5.000 g tablet of benzoic acid (C('HSCOZH) is put into the "bomb" and burned completely in an excess of B water oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is /mu:dmm observed to rise from 14.00 C to 35.47 C over a time of 12.9 minutes. 4 Next, 5.830 g of acetaldehyde (C2H4O) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 14.00 C to 39.15 C. : chemical reaction "bomb" Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: A "bomb" calorimeter. 2C,H,0(g) + 50,(g) 4C0,(g) + 4H,0(g) Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do these experiments sufficiently carefully, and the values you calculate may not exactly match published values for this reaction. () exothermic x10 Is this reaction exothermic, endothermic, or neither? ) endothermic X O neither S If you said the reaction was exothermic or endothermic, calculate the amount of heat that was KJ released or absorbed by the reaction in the second experiment. kJ Calculate the reaction enthalpy AH_ per mole of C,H,0. _ mol For many purposes we can treat nitrogen (Nz) as an ideal gas at temperatures above its boiling point of 196. C. Suppose the temperature of a sample of nitrogen gas is lowered from 62.0 C to 28.0 C, and at the same time the pressure is increased by 5.0%. ) increase x10 Does the volume of the sample increase, decrease, or stay the same? () decrease () stays the same If you said the volume increases or decreases, calculate the percentage change in :I o the volume. Round your answer to the nearest percent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


