Question: A student wants to prepare a buffer solution with pH=5.80 using NaOH and one of the following weak acids given below. How many grams of
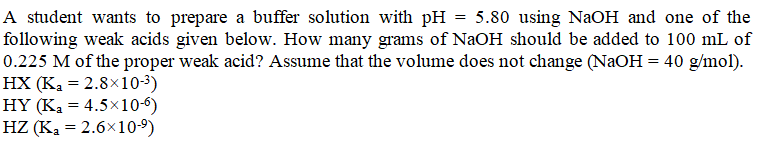
A student wants to prepare a buffer solution with pH=5.80 using NaOH and one of the following weak acids given below. How many grams of NaOH should be added to 100mL of 0.225M of the proper weak acid? Assume that the volume does not change (NaOH=40g/mol). HX(Ka=2.8103) HY(Ka=4.5106) HZ(Ka=2.6109)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


