Question: (a) Suggest a structure for enolate (B) (b) Using cyclic deprotonation models, justify why the enolate (B) has a particular stereochemistry (E or Z). (8
(a) Suggest a structure for enolate (B)
(b) Using cyclic deprotonation models, justify why the enolate (B) has a particular stereochemistry (E or Z). (8 marks)
(c) Using Newman projects, account for why compound (D) has the syn stereochemical relationship between the stereogenic centres labelled 3 and 4. (8 marks)
(d) When the auxiliary is cleaved, will the product compound (E) be a single enantiomer, racemic, or achiral? Justify your answer. (4 marks)
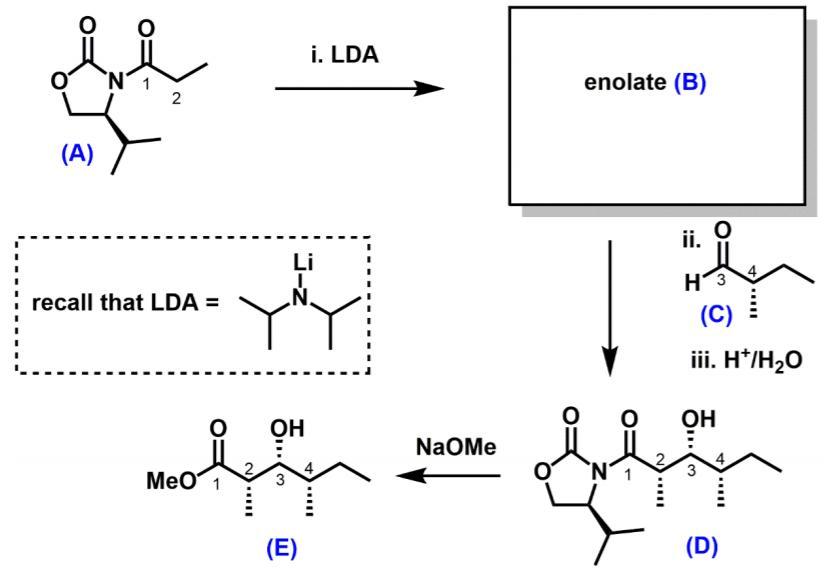
i. LDA N' enolate (B) (A) ii. Li 4 recall that LDA = H3 (C) iii. H*/H20 OH OH NaOMe MeO 1 4 3 (E) (D)
Step by Step Solution
3.55 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


