Question: a The elementary reversible gas phase reaction A + 2B is to be carried out isothermally (250 C) and at constant pressure in a variable
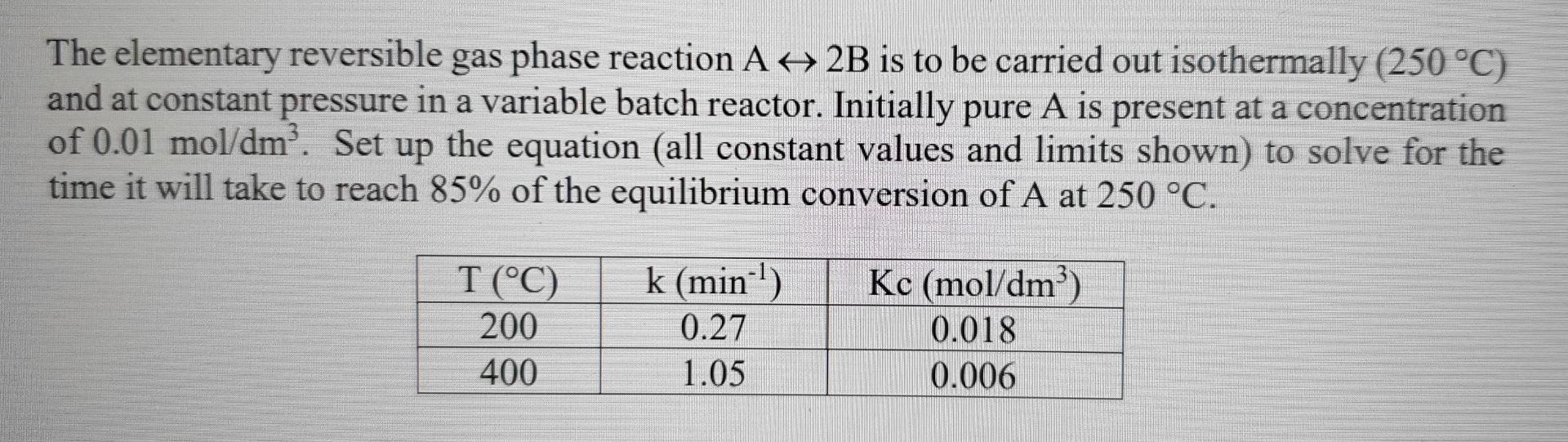
a The elementary reversible gas phase reaction A + 2B is to be carried out isothermally (250 C) and at constant pressure in a variable batch reactor. Initially pure A is present at a concentration of 0.01 mol/dm. Set up the equation (all constant values and limits shown) to solve for the time it will take to reach 85% of the equilibrium conversion of A at 250 C. T(C) 200 400 k (min) 0.27 1.05 Kc (mol/dm) 0.018 0.006
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


