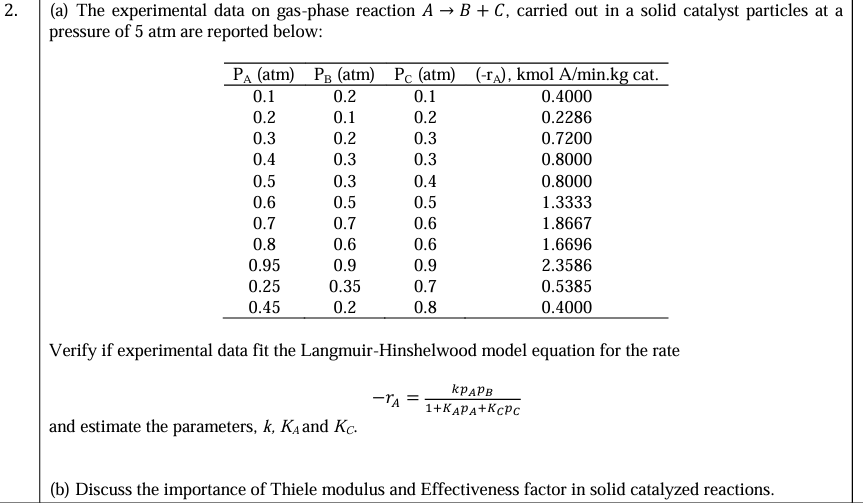
Question: , ( a ) The experimental data on gas - phase reaction A B + C , carried out in a solid catalyst particles at
a The experimental data on gasphase reaction carried out in a solid catalyst particles at a pressure of atm are reported below:
tablePAatmPBatmPCatmrAkmolAminkg cat.
Verify if experimental data fit the LangmuirHinshelwood model equation for the rate
and estimate the parameters, and
b Discuss the importance of Thiele modulus and Effectiveness factor in solid catalyzed reactions.The experimental data on gasphase reaction carried out in a solid catalyst particles at a
pressure of atm are reported below:
PA atm PB atm PC atmrA kmol Aminkg cat.
Verify if experimental data fit the LangmuirHinshelwood model equation for the rate
and estimate the parameters, k KA and KC
b Discuss the importance of Thiele modulus and Effectiveness factor in solid catalyzed reactions.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


