Question: 1. The irreversible first-order gas phase reaction: A - B is carried out in a PBR. Because the reactor is isothermal and total moles don't
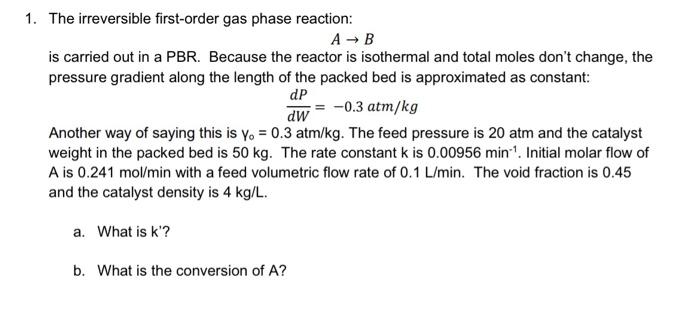
1. The irreversible first-order gas phase reaction: A - B is carried out in a PBR. Because the reactor is isothermal and total moles don't change, the pressure gradient along the length of the packed bed is approximated as constant: dP dW -0.3 atm/kg Another way of saying this is yo = 0.3 atm/kg. The feed pressure is 20 atm and the catalyst weight in the packed bed is 50 kg. The rate constant k is 0.00956 min. Initial molar flow of A is 0.241 mol/min with a feed volumetric flow rate of 0.1 L/min. The void fraction is 0.45 and the catalyst density is 4 kg/L. a. What is k'? b. What is the conversion of A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


