Question: a) The recent problems with Pb2+ release into drinking water from lead pipes in Washington, DC and Flint, MI have to do with water
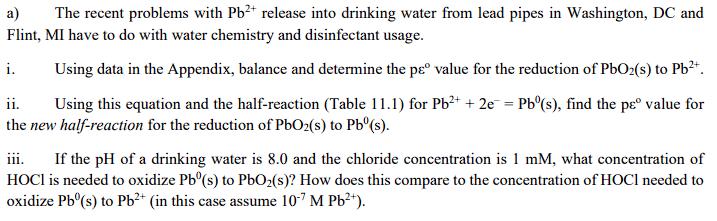
a) The recent problems with Pb2+ release into drinking water from lead pipes in Washington, DC and Flint, MI have to do with water chemistry and disinfectant usage. i. ii. Using data in the Appendix, balance and determine the p value for the reduction of PbO2(s) to Pb2+. Using this equation and the half-reaction (Table 11.1) for Pb2+ + 2e = Pb(s), find the p value for the new half-reaction for the reduction of PbO2(s) to Pb(s). iii. If the pH of a drinking water is 8.0 and the chloride concentration is 1 mM, what concentration of HOCI is needed to oxidize Pb(s) to PbO2(s)? How does this compare to the concentration of HOCI needed to oxidize Pb(s) to Pb2+ (in this case assume 10-7 M Pb2+).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


