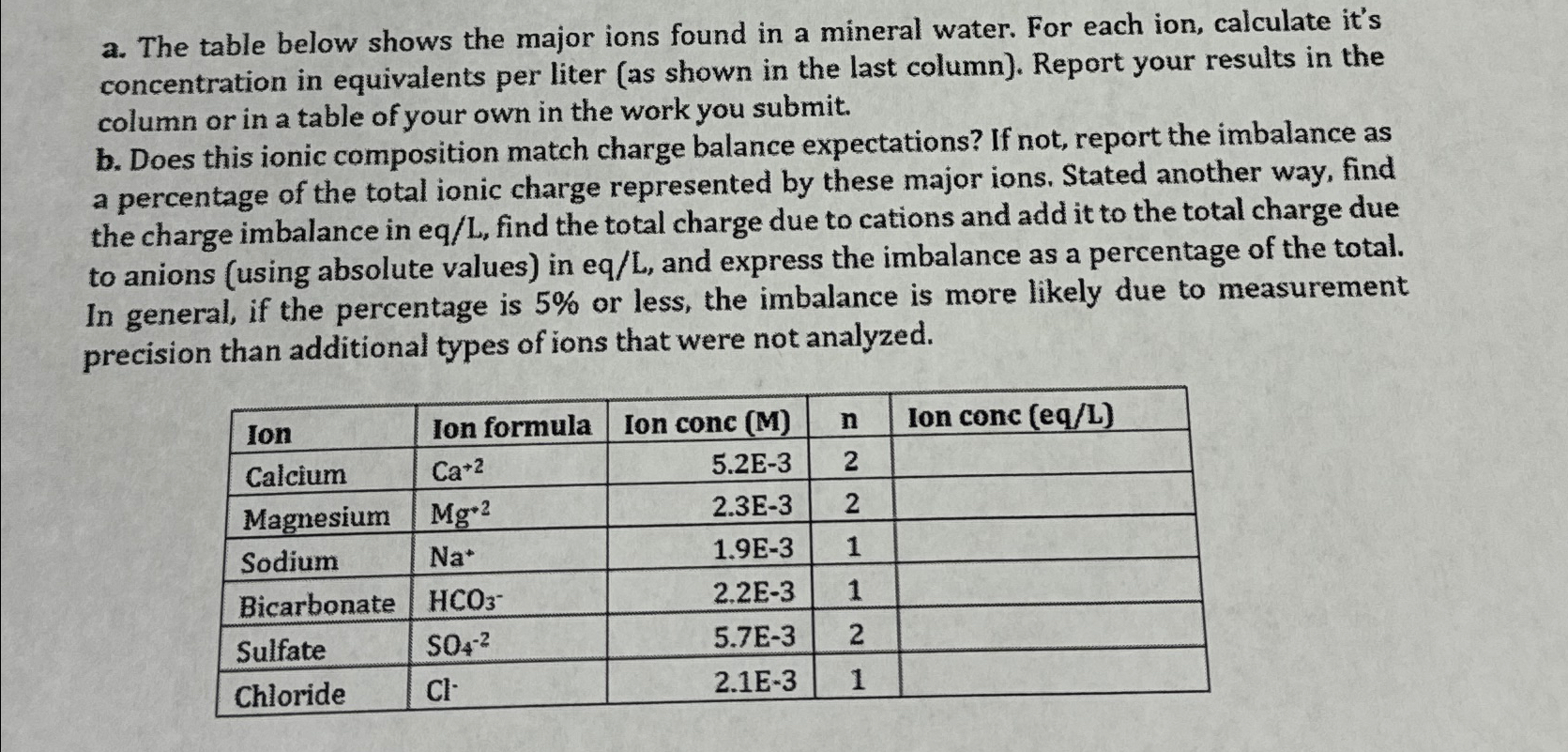
Question: a . The table below shows the major ions found in a mineral water. For each ion, calculate it's concentration in equivalents per liter (
a The table below shows the major ions found in a mineral water. For each ion, calculate it's concentration in equivalents per liter as shown in the last column Report your results in the column or in a table of your own in the work you submit.
b Does this ionic composition match charge balance expectations? If not, report the imbalance as a percentage of the total ionic charge represented by these major ions. Stated another way, find the charge imbalance in eqL find the total charge due to cations and add it to the total charge due to anions using absolute values in eqL and express the imbalance as a percentage of the total. In general, if the percentage is or less, the imbalance is more likely due to measurement precision than additional types of ions that were not analyzed. Please answer the attached question.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


