Question: A thermodynamic system is taken from an initial state i with internal energy U = 100 J to the final state f along two
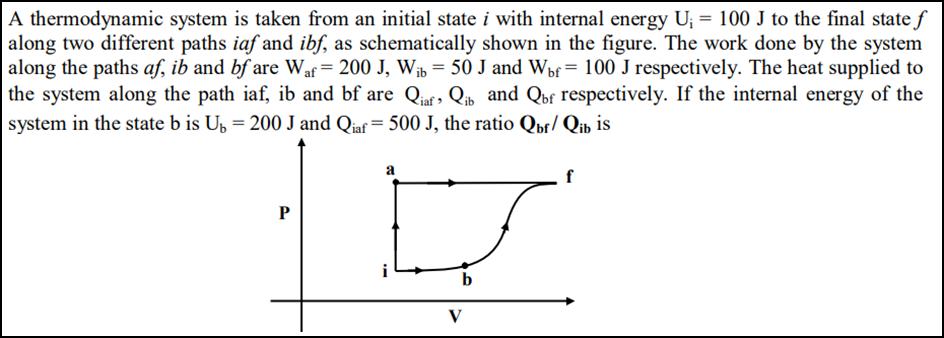
A thermodynamic system is taken from an initial state i with internal energy U = 100 J to the final state f along two different paths iaf and ibf, as schematically shown in the figure. The work done by the system along the paths af, ib and bf are Waf = 200 J, Wib = 50 J and Wbf= 100 J respectively. The heat supplied to the system along the path iaf, ib and bf are Qiaf, Qib and Qbf respectively. If the internal energy of the system in the state b is Ub = 200 J and Qiaf = 500 J, the ratio Qbf/ Qib is P a b V f
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


