Question: A three-stage batch rectifier (first stage is the still pot) is charged with 100kmol of a 20 mol% n-hexane in n-octane mixture. At a

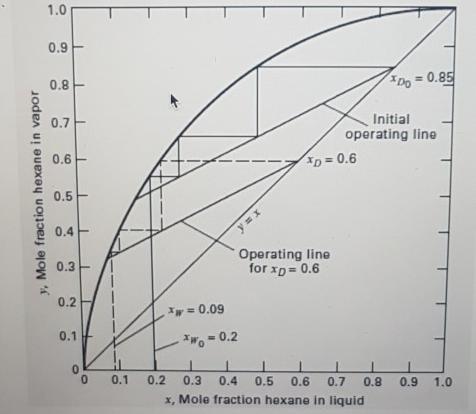
A three-stage batch rectifier (first stage is the still pot) is charged with 100kmol of a 20 mol% n-hexane in n-octane mixture. At a constant-reflux ratio of 1(L/V=0.5), how many moles of charge must be distilled for an average product composition of 70 mol% nC6? The phase equilibrium curve at column pressure is given in Figure below. If the boilup rate is 10 kmol/h, calculate distillation time. 1.0 0.9 0.8 Do = 0.85 0.7 Initial operating line 0.6 D = 0.6 0.5 0.4 Operating line for xp= 0.6 0.3- 0.2 = 0.09 0.1 = 0.2 0.1 0.2 0.3 0.4 0,5 0.6 0.7 0.8 0.9 1.0 x, Mole fraction hexane in liquid y, Mole fraction hexane in vapor
Step by Step Solution
3.39 Rating (143 Votes )
There are 3 Steps involved in it
To solve this problem well utilize the McCabeThiele method to determine the number of moles distilled and the distillation time Step 1 Given Informati... View full answer

Get step-by-step solutions from verified subject matter experts


