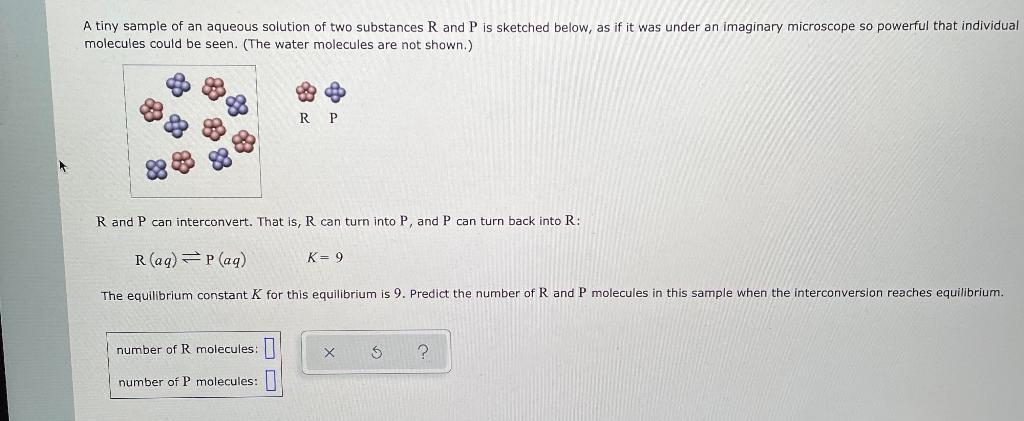
Question: A tiny sample of an aqueous solution of two substances R and P is sketched below, as if it was under an imaginary microscope so
A tiny sample of an aqueous solution of two substances
R
and
P
is sketched below, as if it was under an imaginary microscope so powerful that individual molecules could be seen. (The water molecules are not shown.)
| R | P | ||
R
and
P
can interconvert. That is,
R
can turn into
P
, and
P
can turn back into
R
:
| Raq Paq | =K 9 |
The equilibrium constant
K
for this equilibrium is
9
. Predict the number of
R
and
P
molecules in this sample when the interconversion reaches equilibrium.
|
A tiny sample of an aqueous solution of two substances R and P is sketched below, as if it was under an imaginary microscope so powerful that individual molecules could be seen. (The water molecules are not shown. RP R and P can interconvert. That is, R can turn into P, and P can turn back into R: R(aq) = P(aq) K= 9 The equilibrium constant K for this equilibrium is 9. Predict the number of R and P molecules in this sample when the interconversion reaches equilibrium. X $ ? number of R molecules: 0 number of P molecules: 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


