Question: Initial knowledge Check Question Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen.
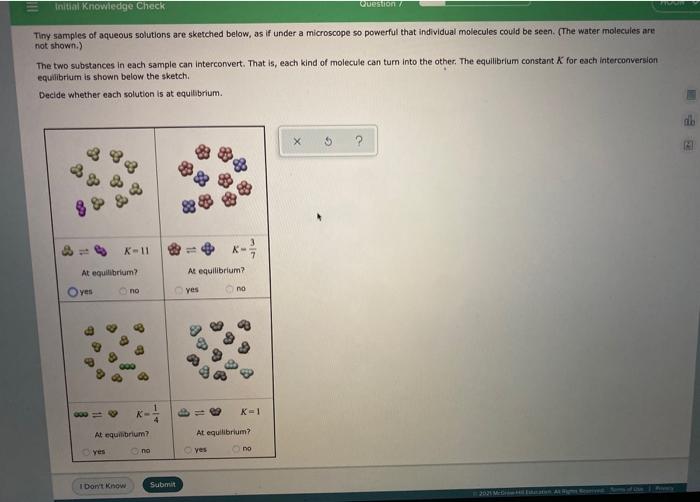
Initial knowledge Check Question Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are not shown.) The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion equilibrium is shown below the sketch Decide whether each solution is at equilibrium X $ K-11 At equilibrium? At equilibrium? Oves no yes no K=1 At equilibrium? At equilibrium? Yes ng yes no I Dont Know Submit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


