Question: A to P second order liquid phase reaction is to be carried out in a jacketed batch reactor for 80% conversion. Initial A composition of
A to P second order liquid phase reaction is to be carried out in a jacketed batch reactor for 80% conversion. Initial A composition of 4 m3 reaction mixture will be 2.3 mol/L. a) Find the necessary reaction time and heat transfer fluid temperature profile (Ts=f(t)) for T=363 K isothermal operation. b) Find the necessary reaction time and temperature profile of the reaction mixture (T=f(t)) under adiabatic conditions, if the initial temperature is To=340 K. c) Discuss all your results and comment on the OTP for this reaction. DATA 1- Heat of reaction : - Delta H= 12,000 cal/mol 2- Heat transfer coefficient for the jacketed reactor: U =130 cal/dm2minoC 3-Avarege physical properties of the mixture: CP =0.95 cal/goC , density =1.02 g/cm3
4- Design graph for the reaction (CA0=2.3 mol/L, Tmax=370 K; rate units [=] mol/Lh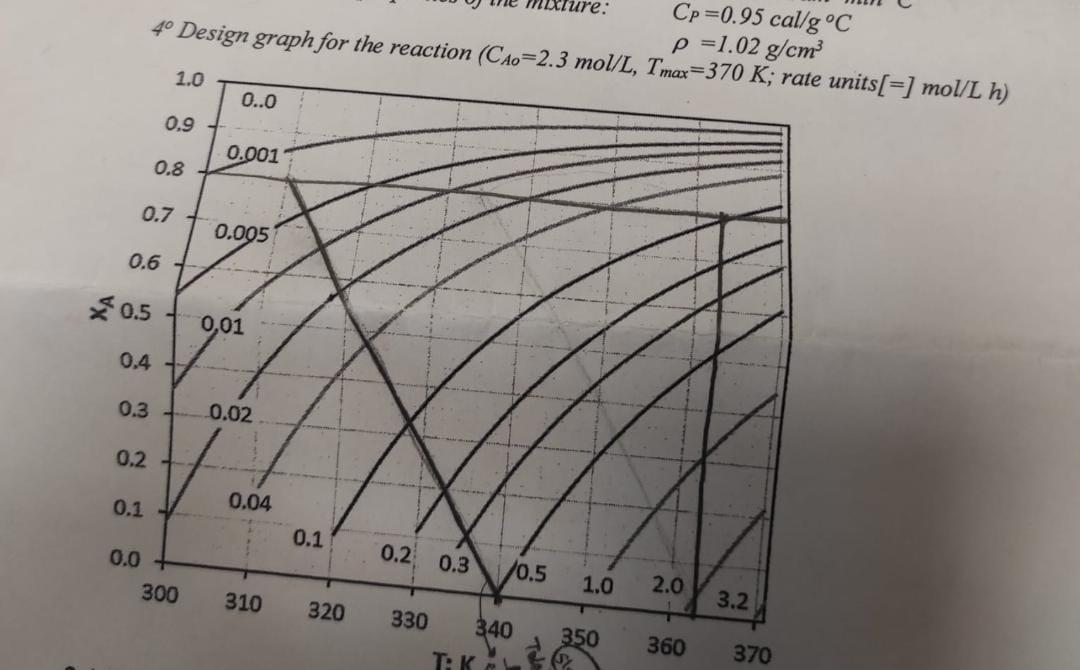
4 Design graph for the reaction (CAn=)3mal/rm=1.02g/cm3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


