Question: a) Use the Euler chain relation with respect to the variables (P,T,V) and an appropriate differentiation to prove that for a van der Waals gas,
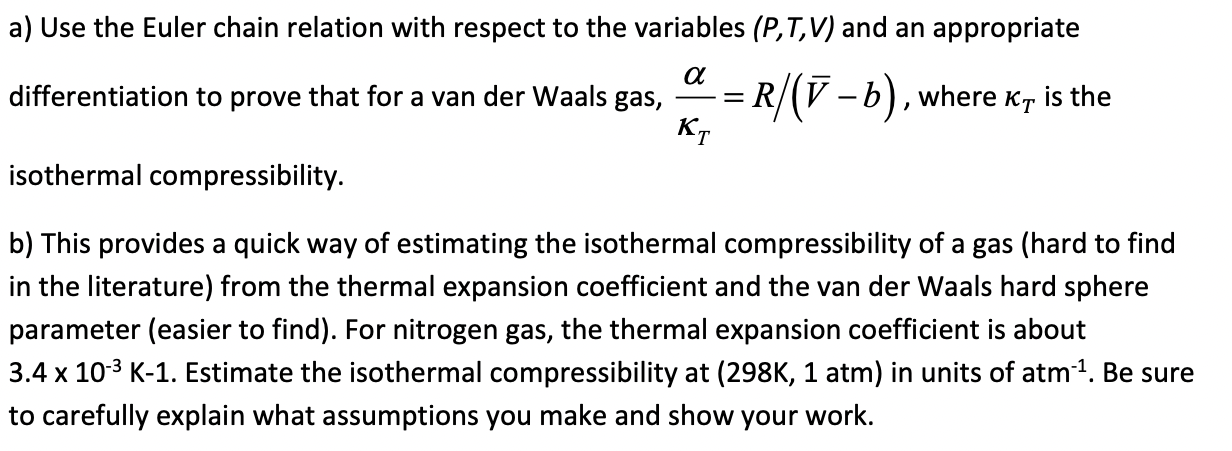
a) Use the Euler chain relation with respect to the variables (P,T,V) and an appropriate differentiation to prove that for a van der Waals gas, T=R/(Vb), where T is the isothermal compressibility. b) This provides a quick way of estimating the isothermal compressibility of a gas (hard to find in the literature) from the thermal expansion coefficient and the van der Waals hard sphere parameter (easier to find). For nitrogen gas, the thermal expansion coefficient is about 3.4103K1. Estimate the isothermal compressibility at (298K,1atm) in units of atm1. Be sure to carefully explain what assumptions you make and show your work
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


