Question: a) Given that the Joule-Thomson coefficient for carbon dioxide is 1.11K atm-1 at (300K, 1.00atm ), look up its heat capacity and calculate (PH)T for
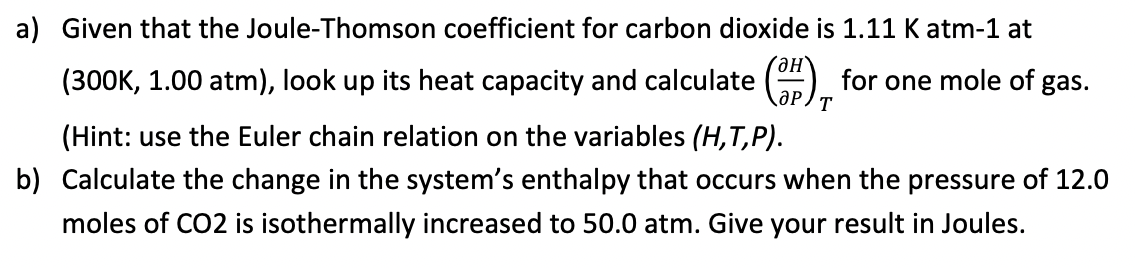
a) Given that the Joule-Thomson coefficient for carbon dioxide is 1.11K atm-1 at (300K, 1.00atm ), look up its heat capacity and calculate (PH)T for one mole of gas. (Hint: use the Euler chain relation on the variables (H,T,P). b) Calculate the change in the system's enthalpy that occurs when the pressure of 12.0 moles of CO2 is isothermally increased to 50.0 atm. Give your result in Joules
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


