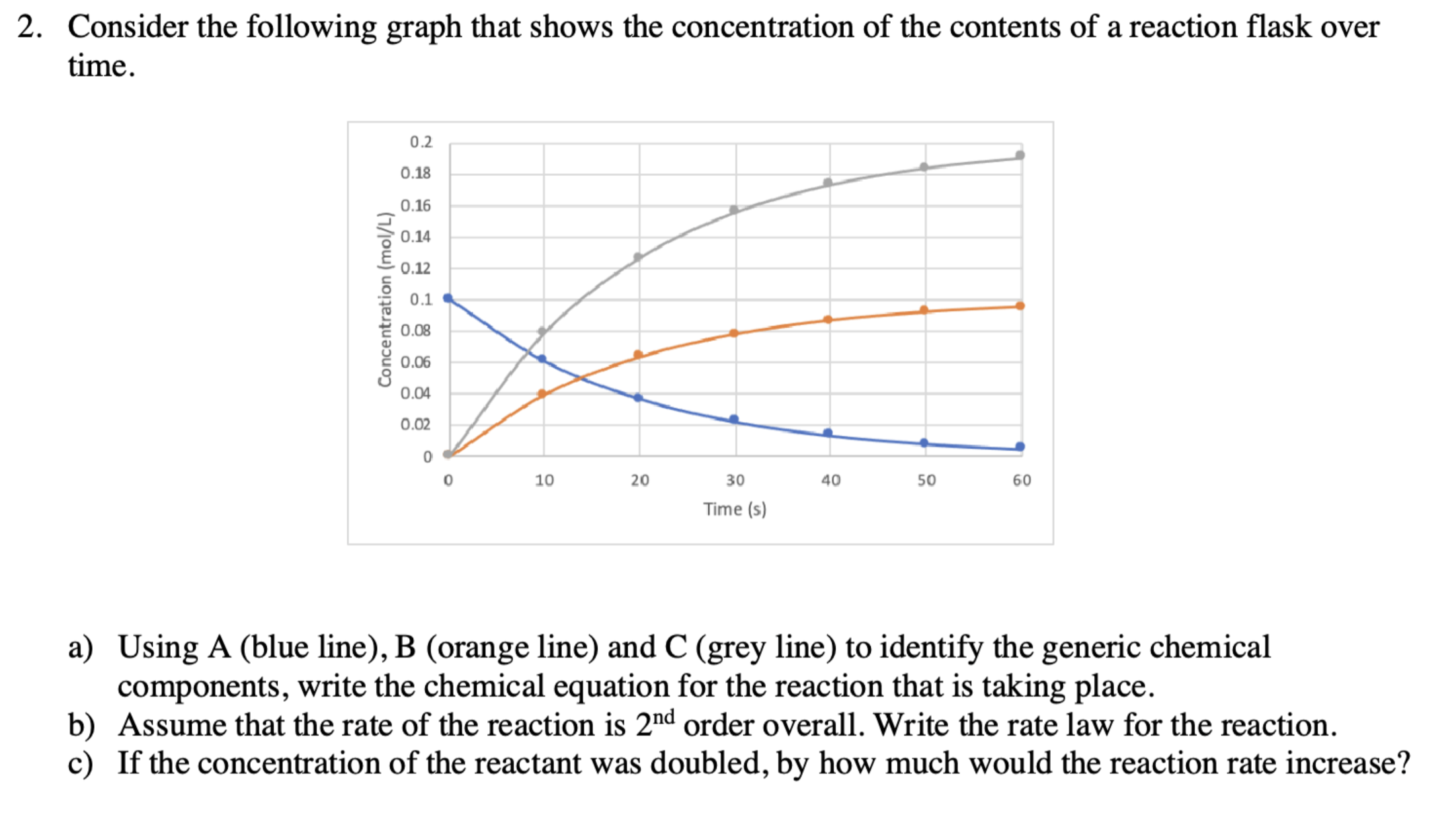
Question: a ) Using A ( blue line ) , B ( orange line ) and C ( grey line ) to identify the generic chemical
a Using A blue line B orange line and C grey line to identify the generic chemical
components, write the chemical equation for the reaction that is taking place.
b Assume that the rate of the reaction is order overall. Write the rate law for the reaction.
c If the concentration of the reactant was doubled, by how much would the reaction rate increase?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


