Question: a. Using your own lab-obtained data, what was your calculated Kc in water? Report your answer to the nearest whole number. b. What is the
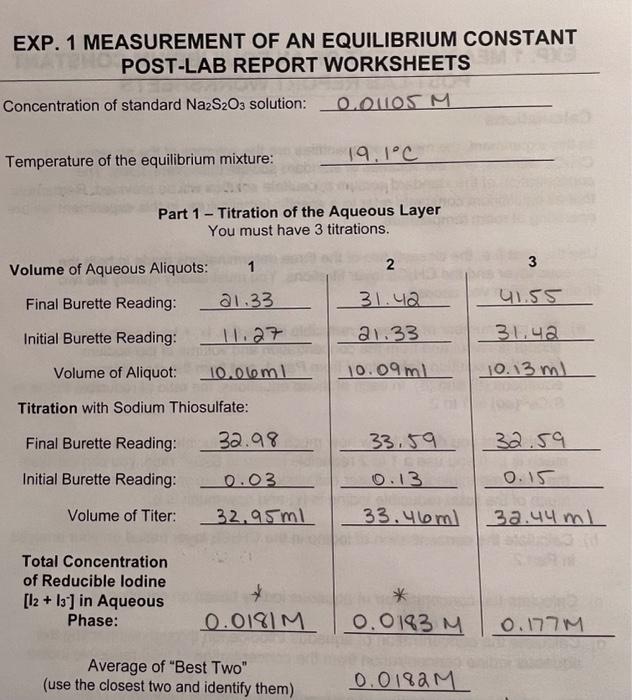
EXP. 1 MEASUREMENT OF AN EQUILIBRIUM CONSTANT POST-LAB REPORT WORKSHEETS Concentration of standard Na2S2O3 solution: 0.01105M Temperature of the equilibrium mixture: 19.1C Part 1 - Titration of the Aqueous Layer You must have 3 titrations. EXP. 1 MEASUREMENT OF AN EQUILIBRIUM CONSTANT POST-LAB REPORT WORKSHEETS Concentration of standard Na2S2O3 solution: 0.01105M Temperature of the equilibrium mixture: 19.1C Part 1 - Titration of the Aqueous Layer You must have 3 titrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


