Question: A vessel containing two binary liquid mixtures of two components (1) and (2) is separated into two chambers by means of a membrane that is
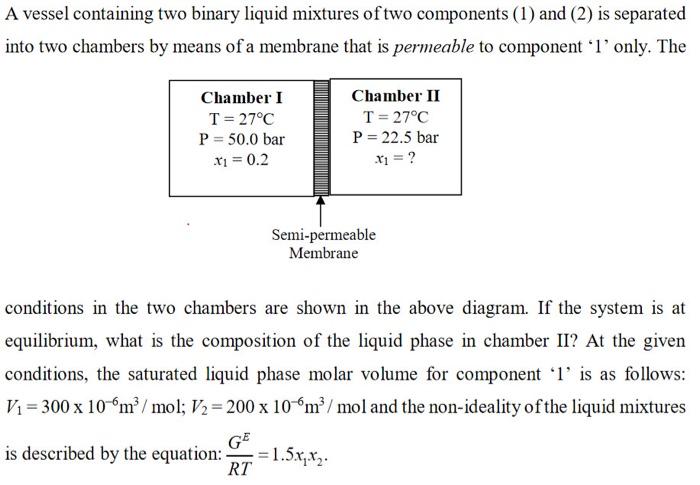
A vessel containing two binary liquid mixtures of two components (1) and (2) is separated into two chambers by means of a membrane that is permeable to component 'l' only. The Chamber I T= 27C P = 50.0 bar x1 = 0.2 Chamber II T=27C P = 22.5 bar x1 = ? Semi-permeable Membrane conditions in the two chambers are shown in the above diagram. If the system is at equilibrium, what is the composition of the liquid phase in chamber II? At the given conditions, the saturated liquid phase molar volume for component l' is as follows: Vi = 300 x 10 m/mol; V2 = 200 x 106m/mol and the non-ideality of the liquid mixtures G is described by the equation: RT = 1.5x,x2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


