Question: A voltaic cell is constructed in which the anode is a ZnZn2+ half cell and the cathode is a CoCo2+ half cell. The half-cell compartments
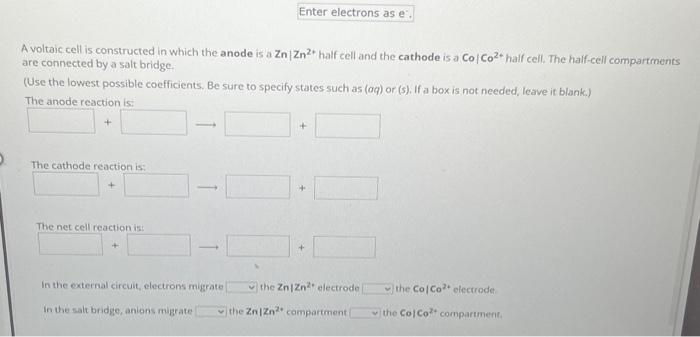
A voltaic cell is constructed in which the anode is a ZnZn2+ half cell and the cathode is a CoCo2+ half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients, Be sure to specify states such as (aq) or (s), If a box is not needed, leave it blank.) The anodereaction is: The cathode reaction is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


