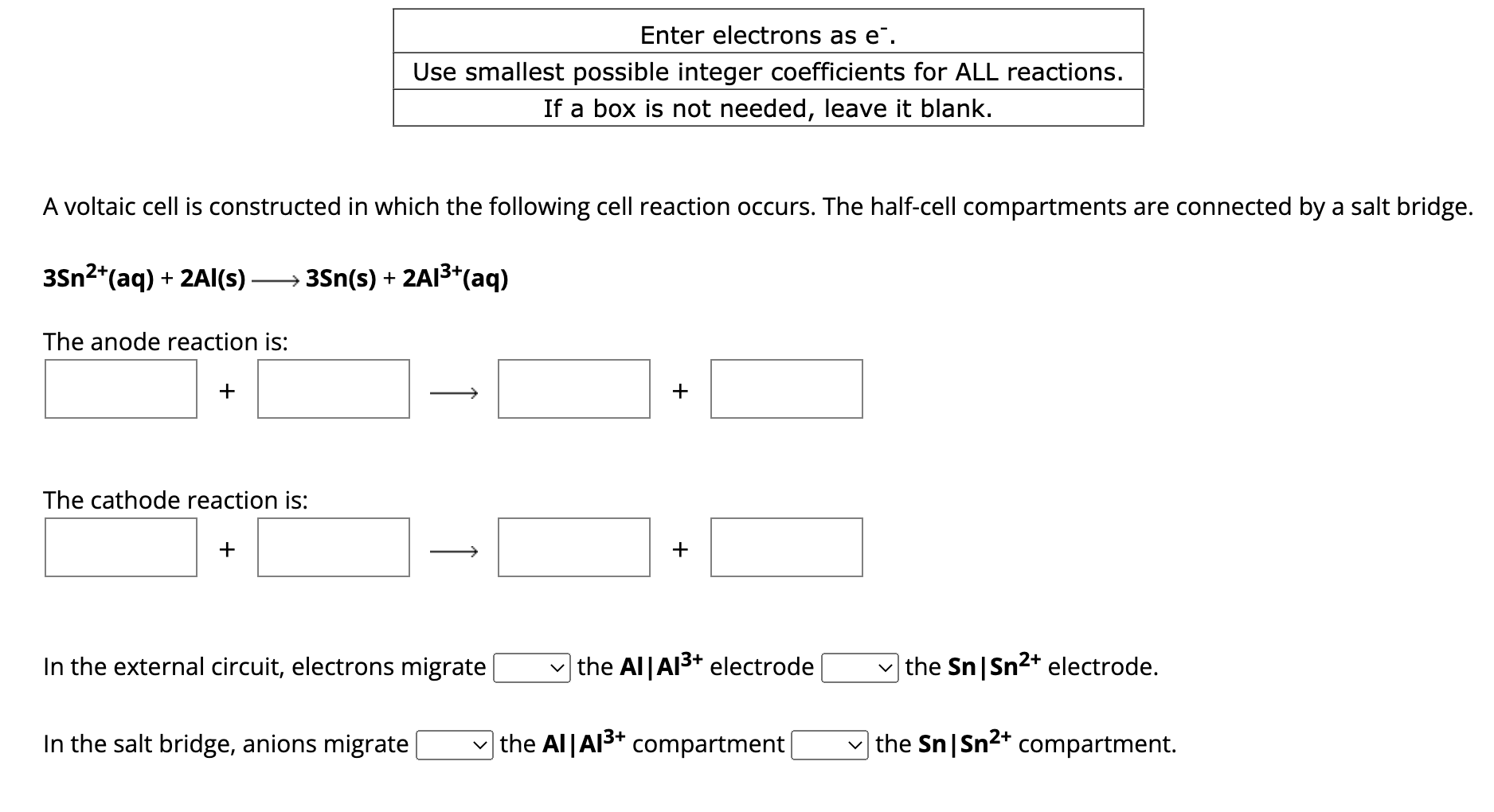
Question: PLEASE HELP, WILL UPVOTE A LOTTT!!!!! A voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a
PLEASE HELP, WILL UPVOTE A LOTTT!!!!!
A voltaic cell is constructed in which the following cell reaction occurs. The half-cell compartments are connected by a salt bridge. 3Sn2+(aq)+2AI(s)3Sn(s)+2AI3+(aq) The anode reaction is: ++ The cathode reaction is: + In the external circuit, electrons migrate the AIA3+ electrode the SnSn2+ electrode. In the salt bridge, anions migrate the AA3+ compartment the SnSn2+ compartment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


