Question: (a) What enthalpy change do H2,H6 and H7 represent? (b) Calculate H5. (ANS : H5=+697L. (c) Calculate the first electron affinity if the second electron
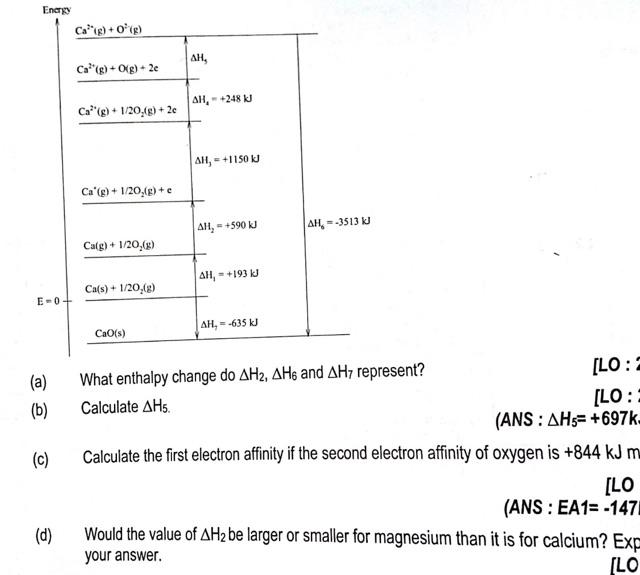
(a) What enthalpy change do H2,H6 and H7 represent? (b) Calculate H5. (ANS : H5=+697L. (c) Calculate the first electron affinity if the second electron affinity of oxygen is +844kJm (ANS:EA1==147 (d) Would the value of H2 be larger or smaller for magnesium than it is for calcium? your
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


