Question: a. What was AT for the water? (AT=Tfinal - Tinitial) b. What was AT for the metal? c. Using the specific heat of water

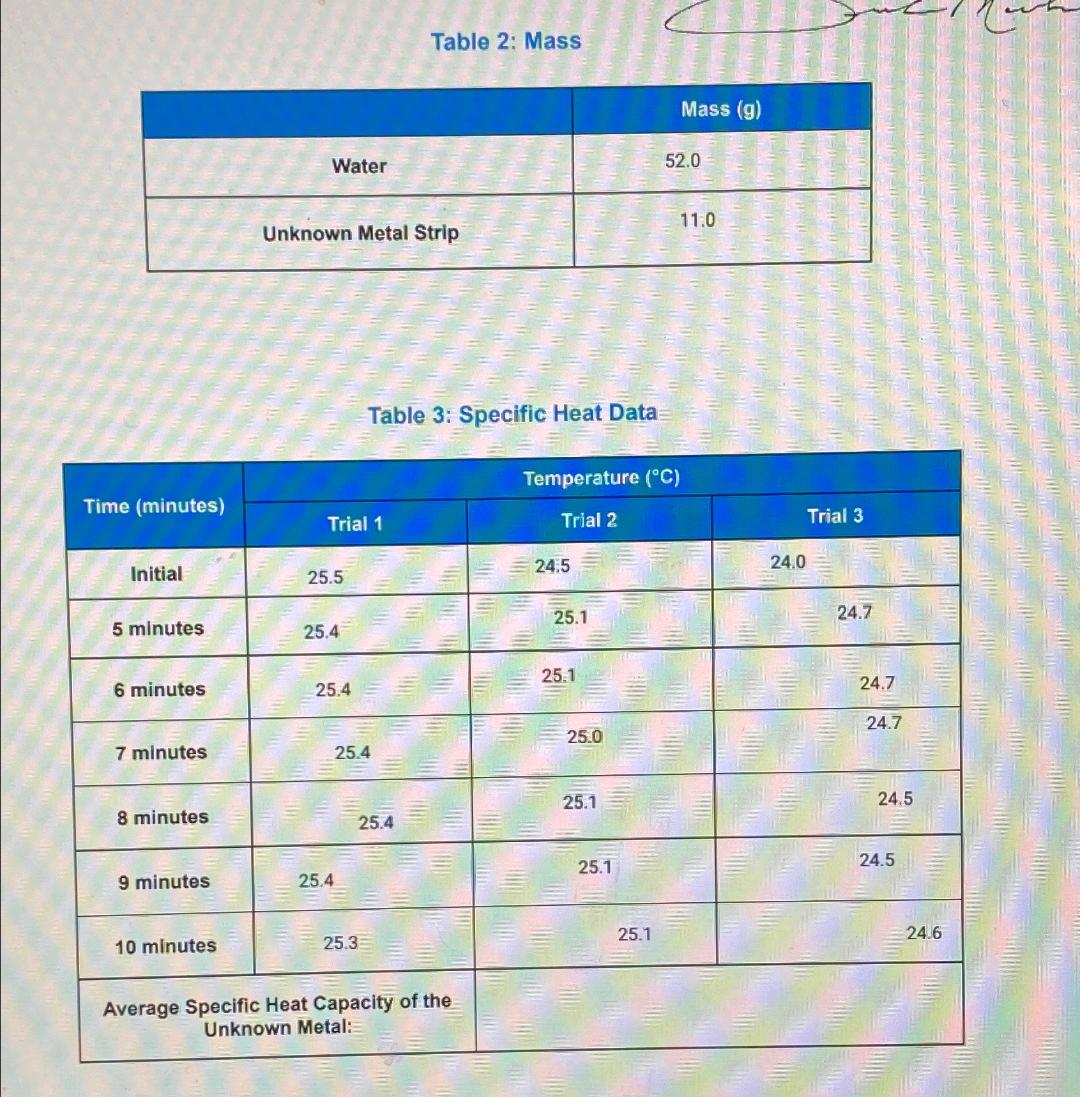
a. What was AT for the water? (AT=Tfinal - Tinitial) b. What was AT for the metal? c. Using the specific heat of water (4.184 J/g C), calculate how much heat flowed into the water d. Calculate the specific heat of the metal. 4. What is the average specific heat capacity of the unknown metal in this experiment? 3 @ C 5 - n E Dj 2 a 5. What is the unknown metal? Use Table 1 for reference. F 11 Water Table 2: Mass Mass (g) 52.0 11.0 Unknown Metal Strip Table 3: Specific Heat Data Temperature (C) Time (minutes) Trial 1 Trial 2 Initial 24.5 25.5 25.1 5 minutes 25.4 25.1 6 minutes 25.4 7 minutes 25.4 8 minutes 9 minutes 25.4 10 minutes 25.3 Trial 3 24.0 24.7 24.7 24.7 25.0 25.1 25.4 Average Specific Heat Capacity of the Unknown Metal: 25.1 25.1 24.5 24.5 24.6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


