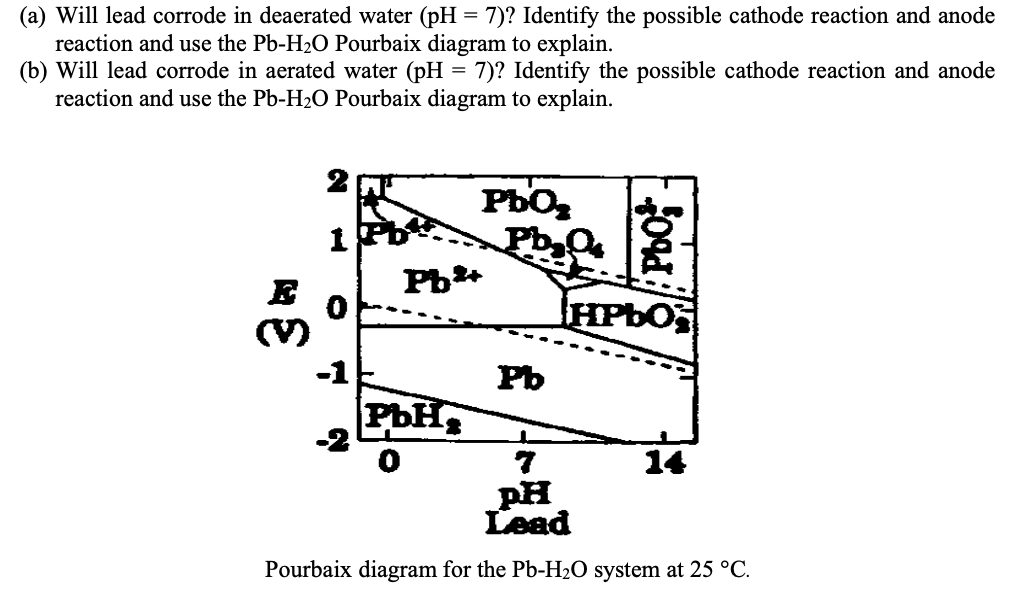
Question: ( a ) Will lead corrode in deaerated water ( p H = 7 ) ? Identify the possible cathode reaction and anode reaction and
a Will lead corrode in deaerated water Identify the possible cathode reaction and anode reaction and use the Pourbaix diagram to explain.
b Will lead corrode in aerated water Identify the possible cathode reaction and anode reaction and use the Pourbaix diagram to explain.
Pourbaix diagram for the system at
Do not use ChatGPT or copy other chegg answers.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


