Question: A Zn wire and Ag/AgCl reference electrode saturated with KCl (E = 0.197 V) are placed into a solution of ZnSO4. The Zn wire is
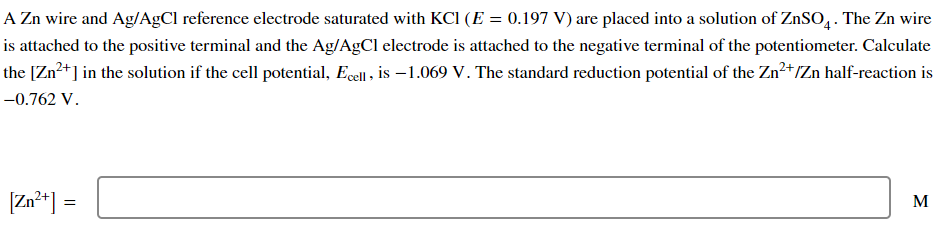
A Zn wire and Ag/AgCl reference electrode saturated with KCl (E = 0.197 V) are placed into a solution of ZnSO4. The Zn wire is attached to the positive terminal and the Ag/AgCl electrode is attached to the negative terminal of the potentiometer. Calculate the [Zn2+] in the solution if the cell potential, Ecell, is 1.069 V. The standard reduction potential of the Zn2+/Zn half-reaction is -0.762 V. [Zn2+] = = M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


