Question: = A+B= 20 Starting with a molar ratio B/A = 0.75 (no diluents) and P = 2 atm in a constant volume isothermal batch reactor:
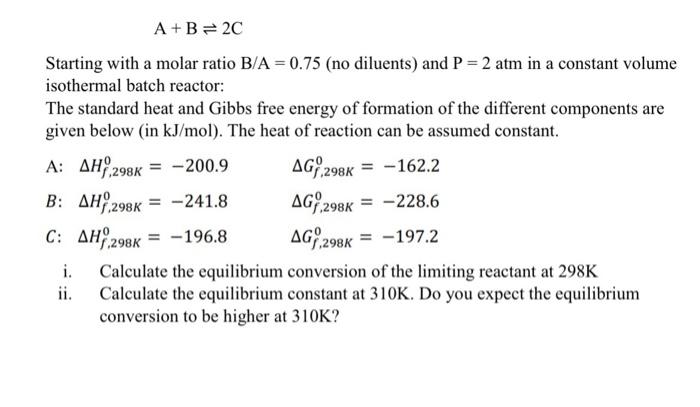
= A+B= 20 Starting with a molar ratio B/A = 0.75 (no diluents) and P = 2 atm in a constant volume isothermal batch reactor: The standard heat and Gibbs free energy of formation of the different components are given below (in kJ/mol). The heat of reaction can be assumed constant. -200.9 AG;,298K = -162.2 = -241.8 = -228.6 = -196.8 = -197.2 i. Calculate the equilibrium conversion of the limiting reactant at 298K ii. Calculate the equilibrium constant at 310K. Do you expect the equilibrium conversion to be higher at 310K? A: AH,298K B: AH 298K C: AH 298K AGY,298K AG%,298K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


