Question: A,B,C,D In Section 11.5 in the textbook we defined the vapor pressure of a liquid in terms of an equilibrium. Write the equation representing the

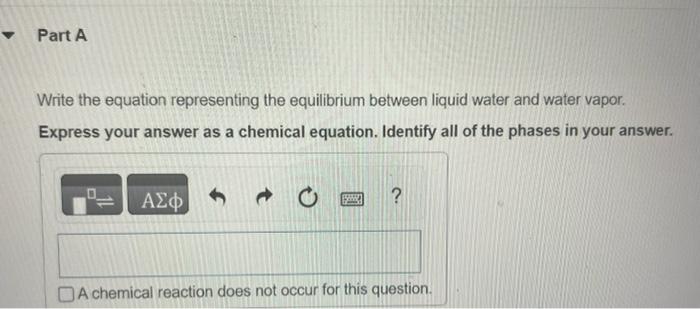
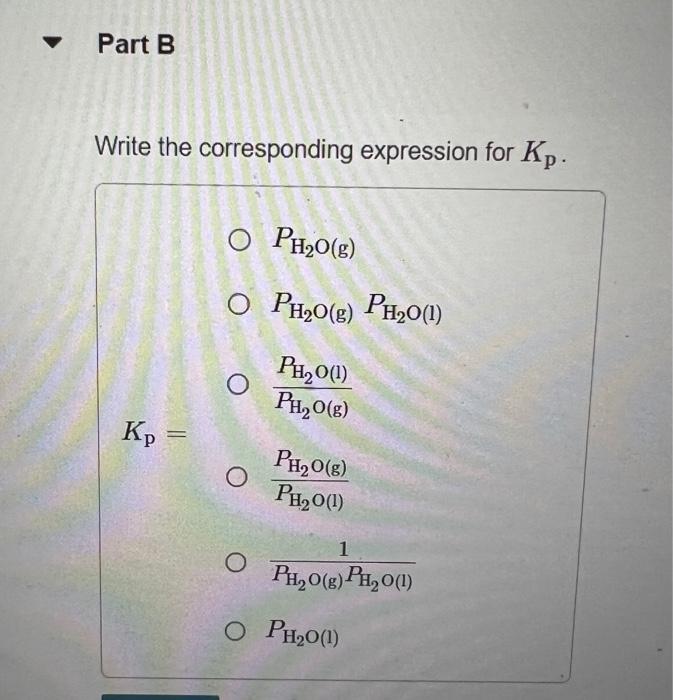
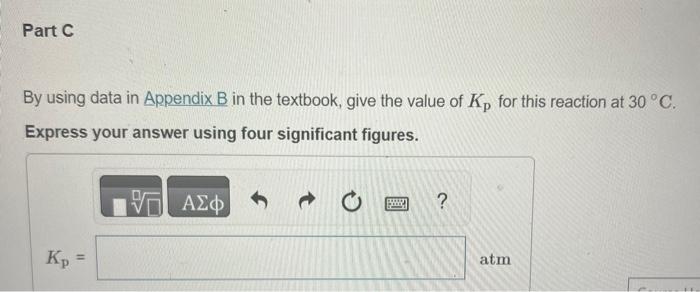

In Section 11.5 in the textbook we defined the vapor pressure of a liquid in terms of an equilibrium. Write the equation representing the equilibrium between liquid water and water vapor. Express your answer as a chemical equation. Identify all of the phases in your answer. Write the corresponding expression for Kp. PH2O(g)PH2O(g)PH2O(l)PH2O(g)PH2O(l)Kp=PH2O(l)PH2O(g)PH2O(g)PH2O(l)1PH2O(l) By using data in Appendix B in the textbook, give the value of Kp for this reaction at 30C. Express your answer using four significant figures. What is the value of Kp for any liquid in equilibrium with its vapor at the normal boiling point of the liquid? Express your answer using three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


