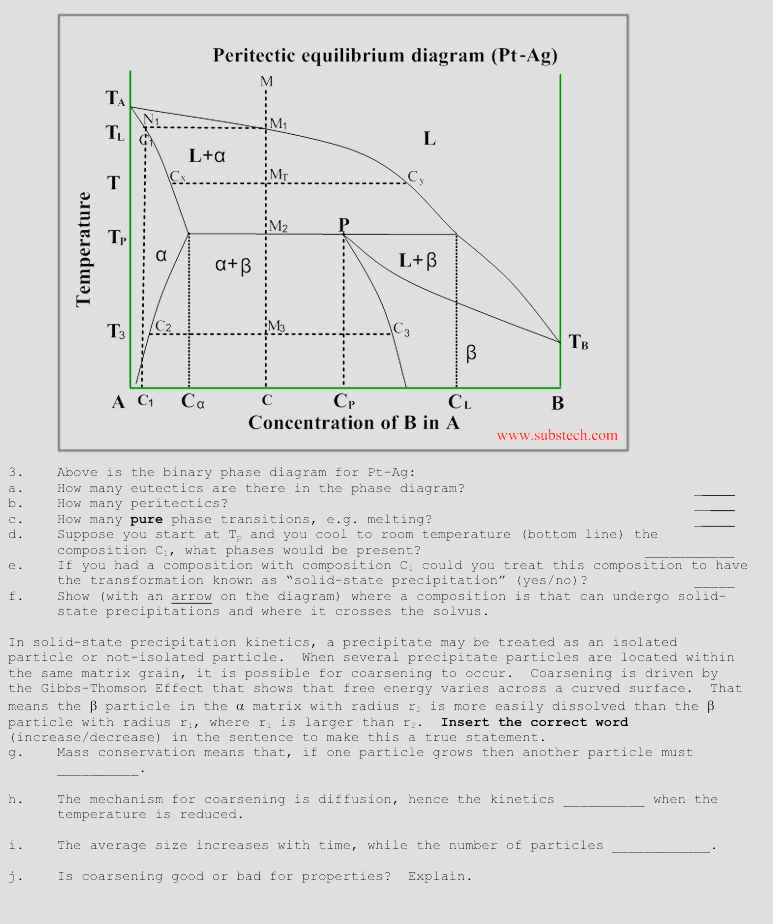
Question: Above is the binary phase diagram for Pt - g: a . How many eutectics are there in the phase diagram? b . How many
Above is the binary phase diagram for Ptg:
a How many eutectics are there in the phase diagram?
b How many peritectics?
c How many pure phase transitions, eg melting?
d Suppose you start at and you cool to room temperature bottom line the
composition what phases would be present?
e If you had a composition with composition could you treat this composition to have
the transformation known as "solidstate precipitation" yesno
f Show with an arrow on the diagram where a composition is that can undergo solid
state precipitations and where it crosses the solvus.
In solidstate precipitation kinetics, a precipitate may be treated as an isolated
particle or notisolated particle. When several precipitate particles are located within
the same matrix grain, it is possible for coarsening to occur. Coarsening is driven by
the GibbsThomson affect that shows that free energy varies across a curved surface. That
means the particle in the matrix with radius is more easily dissolved than the
particle with radius where is larger than Insert the correct word
increasedecrease in the sentence to make this a true statement.
g Mass conservation means that, if one particle grows then another particle must
h The mechanism for coarsening is diffusion, hence the kinetics when the
temperature is reduced.
i The average size increases with time, while the number of particles
j Is coarsening good or bad for properties? Explain.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


