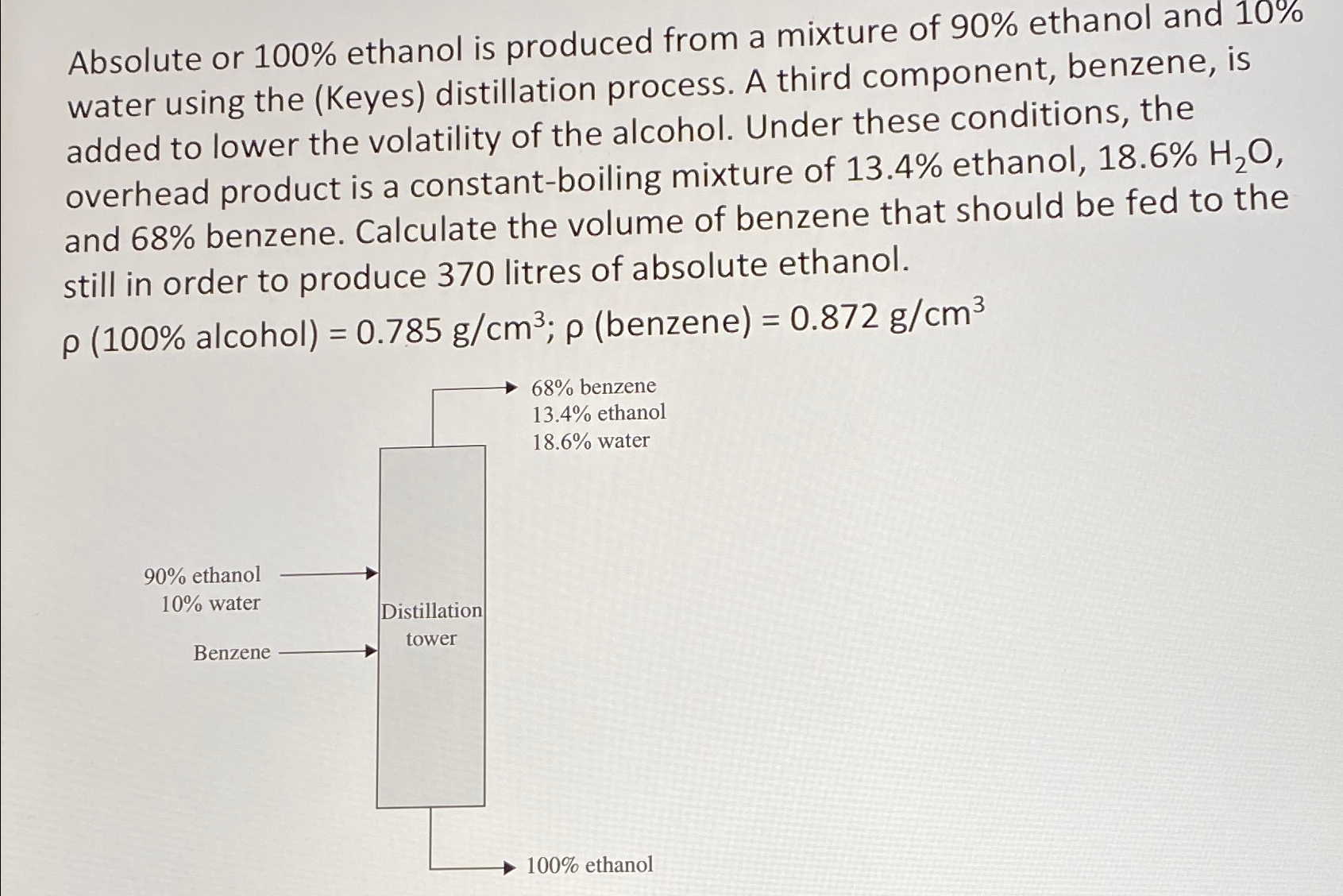
Question: Absolute or 1 0 0 % ethanol is produced from a mixture of 9 0 % ethanol and 1 0 % water using the (
Absolute or ethanol is produced from a mixture of ethanol and water using the Keyes distillation process. A third component, benzene, is added to lower the volatility of the alcohol. Under these conditions, the overhead product is a constantboiling mixture of ethanol, and benzene. Calculate the volume of benzene that should be fed to the still in order to produce litres of absolute ethanol.
alcohol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


