Question: Acetaldehyde ( CH 3 CHO ) is produced by the dehydrogenation of ethanol: C 2 H 5 OH - > CH 3 CHO + H
Acetaldehyde CHCHO is produced by the dehydrogenation of ethanol:
CHOH CHCHO H
An undesired side reaction also occurs and produces ethyl acetate:
CHOH CHCOOCHH
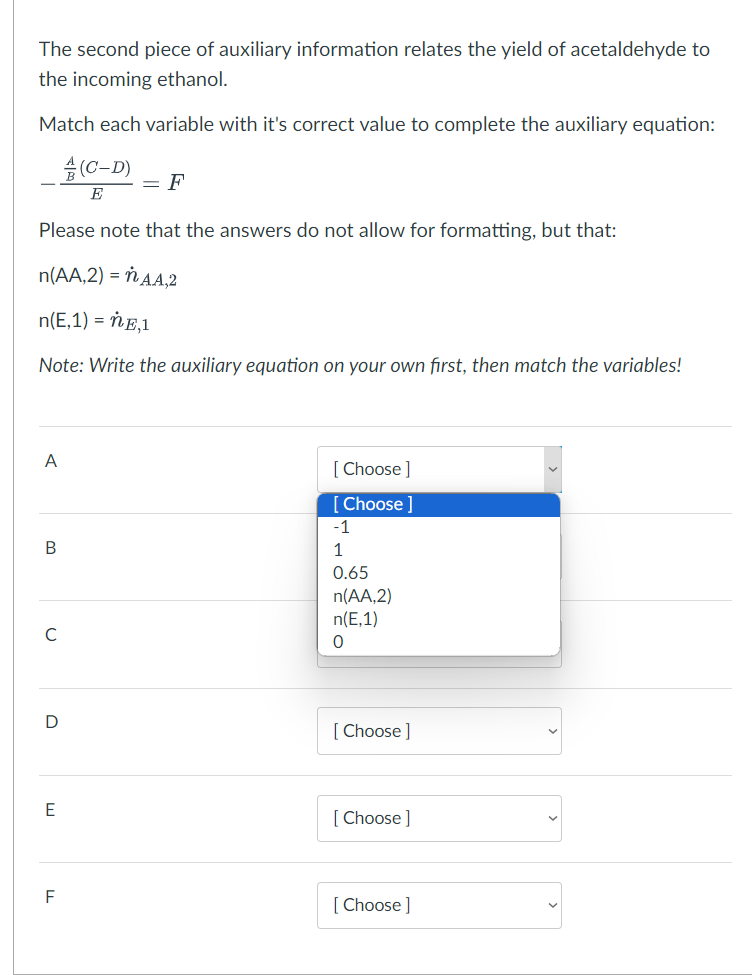
In a pilot plant reactor, of the ethanol fed to the reactor is converted to products, and there is a yield of acetaldehyde based on the total amount of ethanol fed to the reactor
Ethanol vapor enters the reactor at oC bar, and molhr
We want to know how much heat to add or remove in order to keep the outlet temperature at oC
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


