Question: The Arrhenius equation is k = Ae^(-Ea/RT) Where k is the rate constant, E a is the activationenergy, R is the ideal gas constant (8.314
The Arrhenius equation is k = Ae^(-Ea/RT)
Where k is the rate constant, Ea is the activationenergy, R is the ideal gas constant (8.314 J/mole*K) and T is theKelvin temperature.
The slope i got for my trend line is 33.3x+-4.74
and my R2 value is 0.922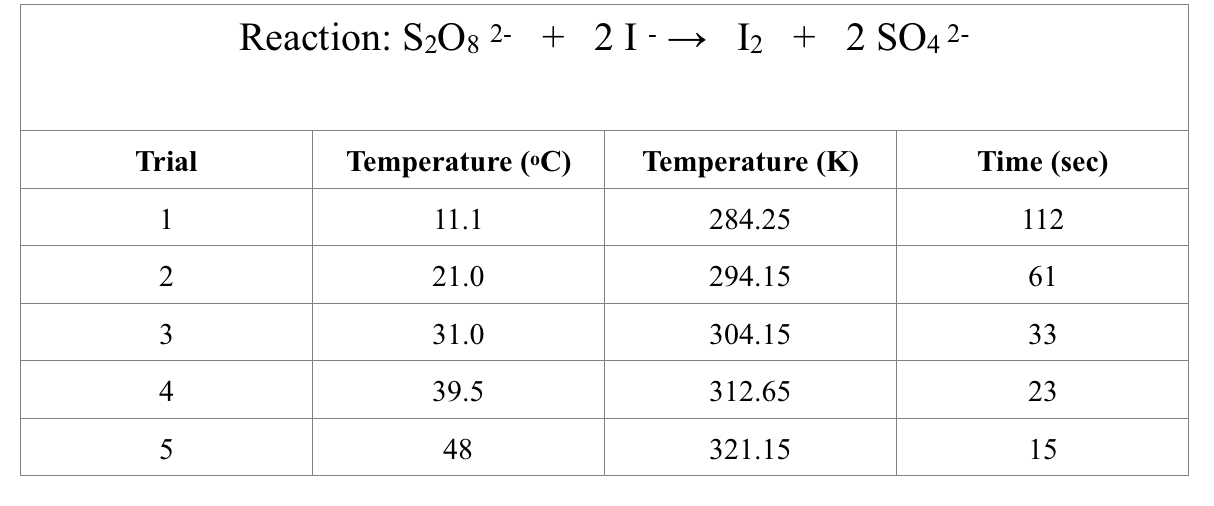
Use the slope of the trendline to calculate the ActivationEnergy (Ea) of the reaction.
Activation Energy =
5. Using your results, calculate how long it would take for thereaction to occur at 0oC.
Time for Reaction to Occur at0oC =______________________________
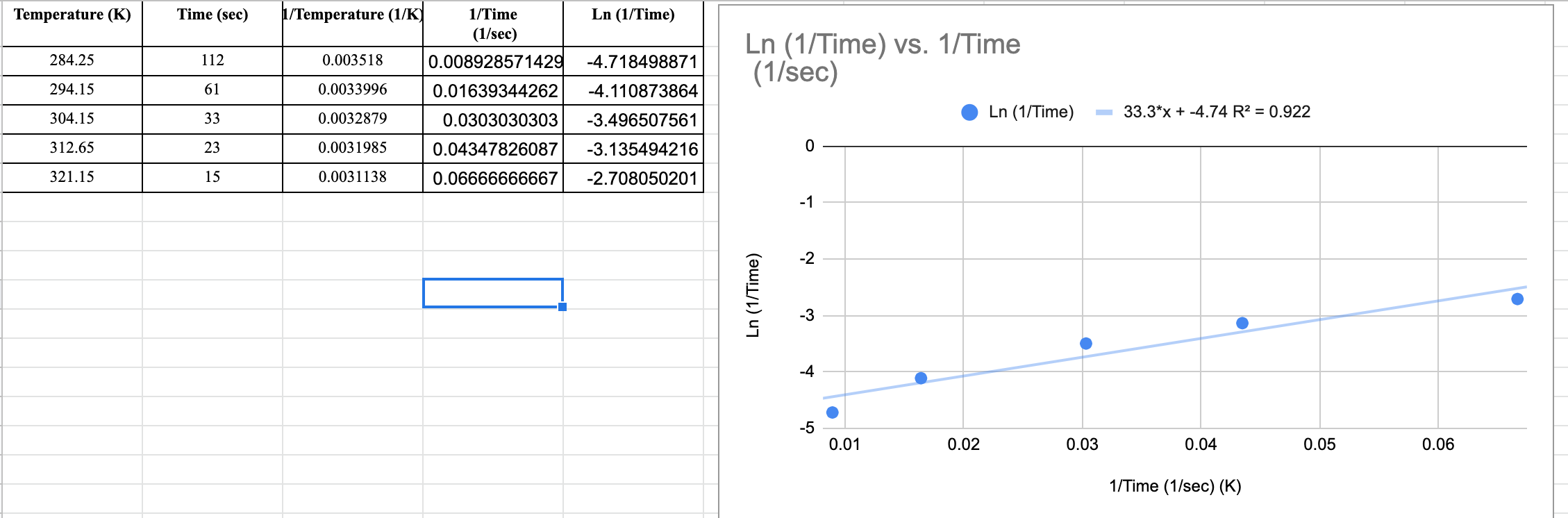
These were the steps needed to be taken. The Y axisneeds to be Ln(1/t) as I made it above
Transfer the time/temperature data to an Excel spreadsheet.
2. Use the operations in Excel to calculate ln(1/?t) and 1/T(remember T must be in Kelvin).
3. Plot ln(1/?t) vs 1/T in a scatter plot format (do not connectthe data points) and apply a linear
Display the trendline, the equation for thetrendline and the R2 value on the graph.
4. Use the slope of the trendline to calculate the ActivationEnergy of the reaction.
Activation Energy and slope. Can someone possibly help solve for this and show work I am having trouble. I calculated for my slopeas seen in the picture.
Temperature (K) 284.25 294.15 304.15 312.65 321.15 Time (sec) 112 61 33 23 15 1/Temperature (1/K) 0.003518 0.0033996 0.0032879 0.0031985 0.0031138 Ln (1/Time) 1/Time (1/sec) 0.008928571429 -4.718498871 0.01639344262 -4.110873864 0.0303030303 -3.496507561 0.04347826087 -3.135494216 0.06666666667 -2.708050201 Ln (1/Time) vs. 1/Time (1/sec) Ln (1/Time) 0 -1 -2 -3 -4 -5 0.01 0.02 Ln (1/Time) 0.03 33.3*x + -4.74 R = 0.922 0.04 1/Time (1/sec) (K) 0.05 0.06
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
To calculate the activation energy Ea of the reaction using the slope of the trendline you can use t... View full answer

Get step-by-step solutions from verified subject matter experts


