Question: Activity 1: Analysis of experimental data The Client has obtained some experimental data from one of their pilot plants, in which n-hexane is chemically transformed
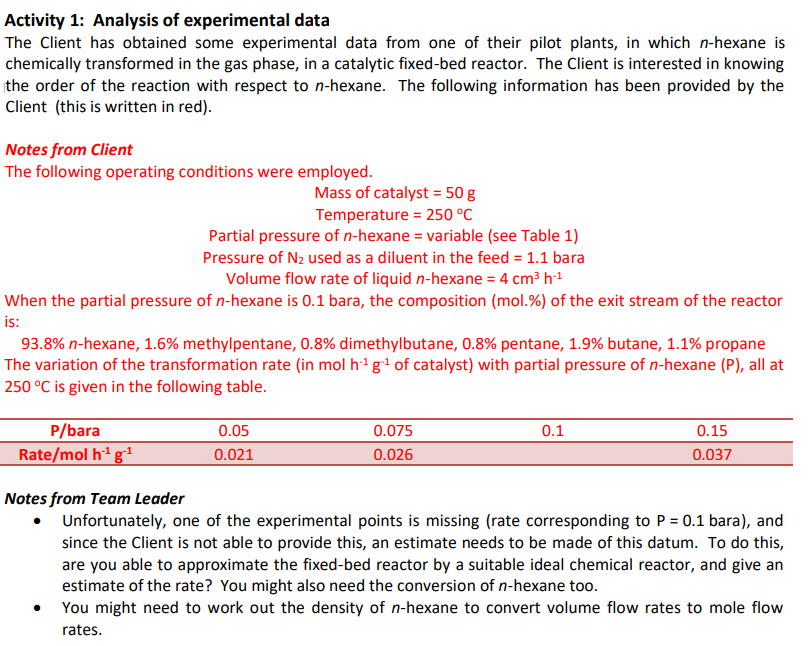
Activity 1: Analysis of experimental data The Client has obtained some experimental data from one of their pilot plants, in which n-hexane is chemically transformed in the gas phase, in a catalytic fixed-bed reactor. The Client is interested in knowing the order of the reaction with respect to n-hexane. The following information has been provided by the Client (this is written in red). Notes from Client The following operating conditions were employed. Mass of catalyst =50g Temperature =250C Partial pressure of n-hexane = variable (see Table 1) Pressure of N2 used as a diluent in the feed =1.1 bara Volume flow rate of liquid n-hexane =4cm3h1 When the partial pressure of n-hexane is 0.1 bara, the composition (mol.\%) of the exit stream of the reactor is: 93.8%n-hexane, 1.6% methylpentane, 0.8% dimethylbutane, 0.8% pentane, 1.9% butane, 1.1% propane The variation of the transformation rate (in molh1g1 of catalyst) with partial pressure of n-hexane (P), all at 250C is given in the following table. Notes from Team Leader - Unfortunately, one of the experimental points is missing (rate corresponding to P=0.1 bara), and since the Client is not able to provide this, an estimate needs to be made of this datum. To do this, are you able to approximate the fixed-bed reactor by a suitable ideal chemical reactor, and give an estimate of the rate? You might also need the conversion of n-hexane too. - You might need to work out the density of n-hexane to convert volume flow rates to mole flow rates
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


