Question: AH -110.2 kJ Given: 4 NO2(g) + O2(g) 2 N2O5(9) find AH for 3 N205(g) 6 NO2(g) + 1.5 02(g). Select one: O A.
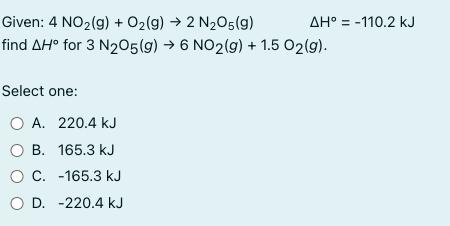
AH -110.2 kJ Given: 4 NO2(g) + O2(g) 2 N2O5(9) find AH for 3 N205(g) 6 NO2(g) + 1.5 02(g). Select one: O A. 220.4 kJ B. 165.3 kJ C. -165.3 kJ O D. -220.4 kJ
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


