Question: all 3 go together please help me answer them Considering the reaction AkbkaB A temperature jump experiment is performed where kaa=296s1 and kbb=300s1, what is
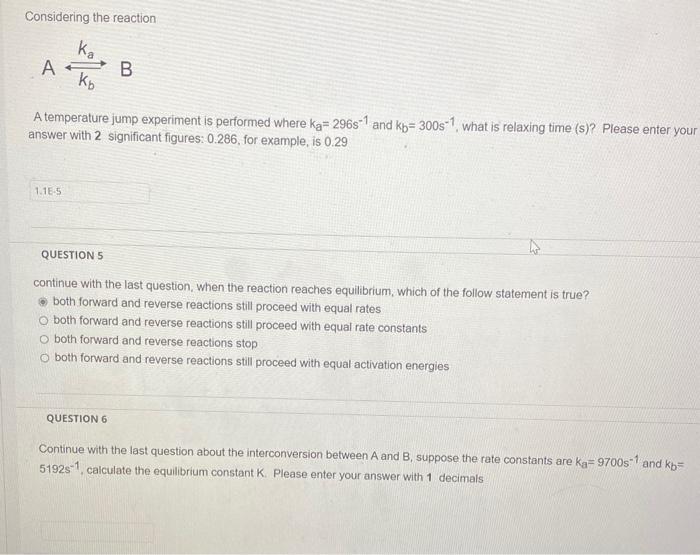
Considering the reaction AkbkaB A temperature jump experiment is performed where kaa=296s1 and kbb=300s1, what is relaxing time (s)? Please enter your answer with 2 significant figures: 0.286, for example, is 0.29 QUESTION 5 continue with the last question, when the reaction reaches equilibrium, which of the follow statement is true? both forward and reverse reactions still proceed with equal rates both forward and reverse reactions still proceed with equal rate constants both forward and reverse reactions stop both forward and reverse reactions still proceed with equal activation energies QUESTION 6 Continue with the last question about the interconversion between A and B, suppose the rate constants are Kaa=9700 s 1 and Kbb= 5192s1, calculate the equilibrium constant K. Please enter your answer with 1 decimals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


