Question: All the transitions that involve the electron transitions either absorptions or emissions which start or end at the energy shell n=1 are called the Lyman
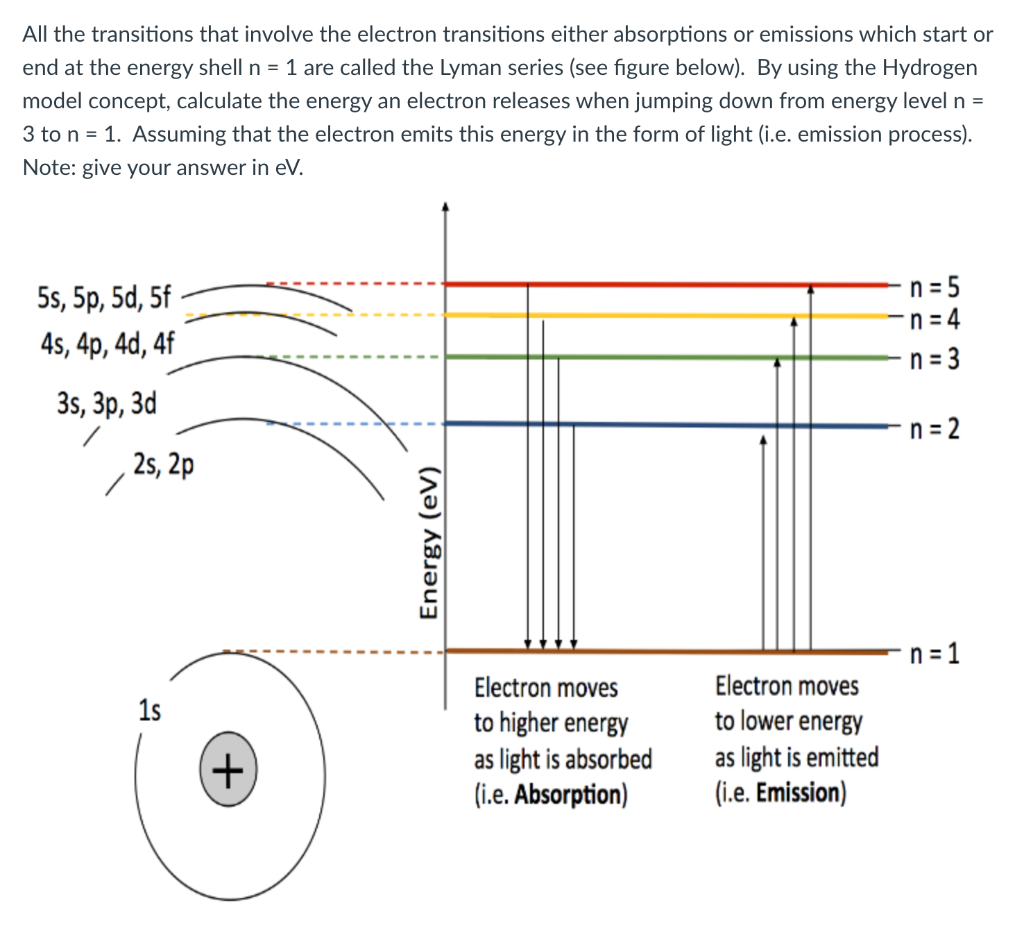
All the transitions that involve the electron transitions either absorptions or emissions which start or end at the energy shell n=1 are called the Lyman series (see figure below). By using the Hydrogen model concept, calculate the energy an electron releases when jumping down from energy level n= 3 to n=1. Assuming that the electron emits this energy in the form of light (i.e. emission process). Note: give your answer in eV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


