Question: All values except -0.526 are incorrect. Please solve to the best of your ability. Methanol (C) is formed from carbon monoxide (A) and hydrogen (B)


All values except -0.526 are incorrect. Please solve to the best of your ability.
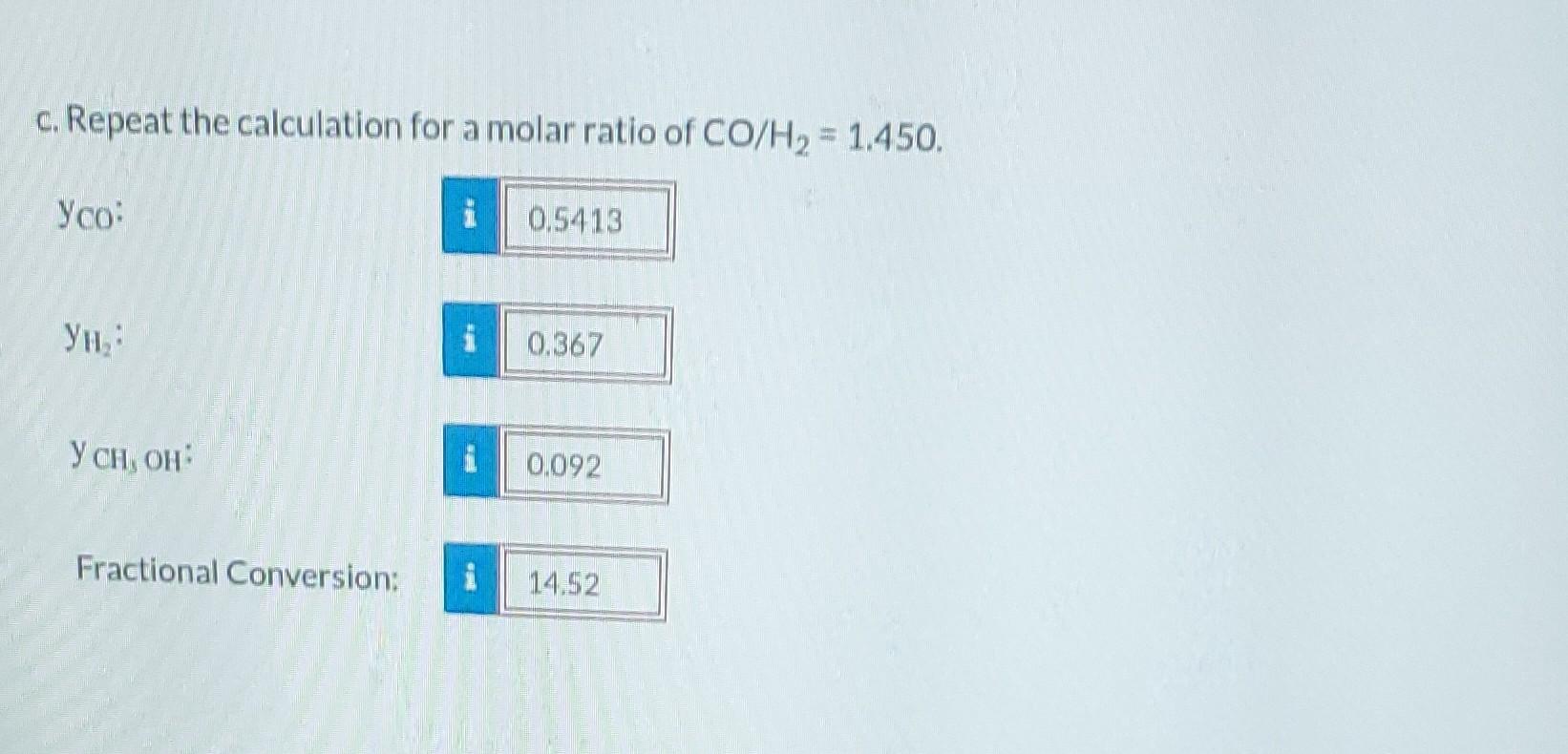
Methanol (C) is formed from carbon monoxide (A) and hydrogen (B) in the gas-phase reaction CO+2H CH3OH The mole fractions of the reactive species at equilibrium satisfy the relation Ye 1 = Keq (T) YA Y P where P is the total pressure (atm), Keg the reaction equilibrium constant (atm-2), and T the temperature (K). The equilibrium constant Keq equals 10.5 at 373 K, and 2.316 x 10-4 at 573 K. A semilog plot of Keq (logarithmic scale) versus 1/T (rectangular scale) is approximately linear between T = 300 K and T = 600 K. a. What is the value of log10(Keqx (1 atm)) at T=422 K? -0.526 b. Suppose you begin with a molar ratio of CO/H = 1.200 and no methanol. If the reaction proceeds to equilibrium at 422 K and 2 atm, calculate the molar composition of the product and the fractional conversion of CO. : 0.468 , Mr 0.425 b. Suppose you begin with a molar ratio of CO/H = 1.200 and no methanol. If the reaction proceeds to equilibrium at 422 K and 2 atm, calculate the molar composition of the product and the fractional conversion of CO. Yco: i 0.468 , 0.425 i 0.1066 0.1855 Y CH;OH Fractional Conversion: c. Repeat the calculation for a molar ratio of CO/H = 1.450. : 1 0.5413 0.367 0.092 14.52 , : Fractional Conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


